
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
From the given structure, indicate the most stable radical.
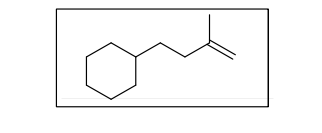
Transcribed Image Text:The image shows the chemical structure of the molecule 4-methylcyclohexene.
**Description:**
- **Cyclohexane Ring:** The diagram begins with a six-membered carbon ring, known as a cyclohexane. It’s represented as a hexagon.
- **Double Bond:** There is a single double bond extending from the cyclohexane to form an alkene, indicated by two parallel lines, showing unsaturation in the molecule.
- **Methyl Group:** Attached to the carbon adjacent to the double bond is a methyl group (CH₃), represented by a line extending from the ring to a connected "V" shape.
This structure illustrates an organic compound that is part of the alkene family, characterized by the presence of a carbon-carbon double bond, and is specifically substituted with a methyl group.
Expert Solution
arrow_forward
Step 1
Organic reactions are those in which organic reactant react to form organic products. Free radical is formed by the homolytic of carbon hydrogen bond. In the given question, it is asked to indicate the most stable radical of given structure.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the equation for the following proton-transfer reaction by using curved arrows to show the flow of electron pairs and drawing the products of the reaction. • Draw all atoms, including hydrogen atoms. Apply formal charges where appropriate. Assign lone pairs and radical electrons where appropriate. • Use the "starting points" menu to revert to the original molecule(s) shown. Draw the appropriate electron-flow arrows. . Omit + signs between structures. ● ● ● H H ││ H-C-C-0: T I H H == starting points == C H-Cl: در ? ChemDoodlearrow_forwardPlease explain Consider the reaction between methylcyclohexene and the hydroxyl radical.arrow_forwardDRAW THE PROPAGATION STEPS WITH PROPER ELECTRON FLOW ARROWS FOR THE FOLLOWING REACTION. Br-Br Br H-Brarrow_forward
- Please don't provide handwriting solutionarrow_forwardverse osis Consider the structures of the carbocations formed by ortho attack of the electrophile, *NO₂, on the given starting material. NH2 Part: 0/2 Part 1 of 2 + Draw all resonance structures for the carbocation formed by ortho attack of the electrophile "NO, on the given starting material. If applicable, include the resonance structure in which π bond electrons "move" to the more electronegative atom as a lone pair. Be sure to include all charges and relevant lone pairs. 00 al. Ar B Karrow_forwardplz do both with detail explanation plz ...do not give incomplete solutionarrow_forward
- O Macmillan Learning Question 21 of 35 Draw the correct product for the Diels-Alder reaction. > + Select Drarrow_forwardWhich of the three main parts of a radical reaction does the following reaction represent?arrow_forward2ClF+O2>>>Cl2O+F2O delta H=167.4kJ/mol 2ClF3+2O2>>>Cl2O+3F2O deltaH=341.4kJ/mol 2F2+O2>>>2F2O Delta H= -43.4kJ/mol Calculate delta H for the reaction ClF3>>>ClF+F2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY