
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
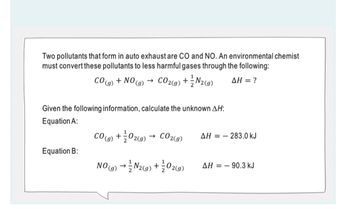
Transcribed Image Text:Two pollutants that form in auto exhaust are CO and NO. An environmental chemist
must convert these pollutants to less harmful gases through the following:
CO2(g) + N2(g) ΔΗ = ?
CO(g)
Equation B:
+ NO ->
(9)
Given the following information, calculate the unknown AH:
Equation A:
1
CO(g) + 02(g)
CO2(g)
NO(g) → N₂(g) + O2(9)
AH=283.0 kJ
AH 90.3 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The reaction between nitrogen and oxygen gases requires the absorption of 43 kJ of energy per mole of nitrogen according to the following chemical equation, N2(g) + O2(g) → 2 NO(g). How many grams of NO are produced in the reaction if 417 kJ of heat are absorbed by the reaction?arrow_forwardA chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 4.40kg of water at 30.9°C. During the reaction 130.kJ of heat flows out of the flask and into the bath.Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18·J·g−1K−1. Be sure your answer has the correct number of significant digitsarrow_forwardConsider the two diagrams showing the energies (boxes) of each of four A particles and four B particles shown below. The dotted lines represent the allowed energies of each particle. Please note that the horizontal lines of the graphs represent possible energy levels for molecules. Do not give handwriting solution.arrow_forward
- The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. It's also how you can make sodium carbonate, another useful chemical, also called washing soda. The balanced equation for the decomposition of sodium bicarbonate into sodium carbonate, carbon dioxide, and water is: 2 NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g) When 29.27 grams of NaHCO3 decomposes what is the volume of CO2 formed at stp?arrow_forwardIn the following equation for a chemical reaction, the notation (s), (1), or (g) indicates whether the substance indicated is in the solid, liquid, or gaseous state. CH4(g) + H,0(g) + energy → 3H2(g) + CO(g) Identify each of the following as a product or a reactant: H,O(g) H,(g) CHĄ(g) CO(g) When the reaction takes place energy is The reaction isarrow_forwardA chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 6.90kg of water at 32.2°C. During the reaction 126.kJ of heat flows out of the flask and into the bath. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18·J·g−1K−1. Round your answer to 3 significant digits.arrow_forward
- Titanium reacts with iodine to form titanium(III) iodide, emitting heat, via the following reaction: 2Ti(s)+3I2(g)→2TiI3(s), ΔHrxn=−839kJ Determine the mass of titanium that reacts if 1.53×103 kJ of heat is emitted by the reaction. Express your answer to three significant figures and include the appropriate units. Determine the mass of iodine that reacts if 1.53×103 kJ of heat is emitted by the reaction. Express your answer to three significant figures and include the appropriate units.arrow_forwardA chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 3.50 kg of water at 38.6 °C. During the reaction 131. kJ of heat flows out of the flask and into the bath.Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18·J·g^−1K^−1. Round your answer to 3 significant digits.arrow_forwardConsider these reactions, where M represents a generic metal. 1. 2 M(s) + 6 HCI(aq) 2 MCI, (аq) + 3 Н, (2) AH1 = -556.0 kJ 2. HCl(g) HCl(aq) AĦ2 = -74.8 kJ 3. H, (g) + Cl, (g) → 2 HCI(g) ΔΗ = -1845.0 kJ 4. MCI, (s) – MCI, (aq) AĦ4 = -493.0 kJ Use the given information to determine the enthalpy of the reaction 2 M(s) + 3 Cl, (g) – - 2 MCl, (s) -556.0 ΔΗ- Incorrectarrow_forward
- A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 2.70kg of water at 39.2°C . During the reaction 128.kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18·J·g−1K−1 . Be sure your answer has the correct number of significant digits.arrow_forwardWhen 145 mL of 0.212 M NaCl(aq) and 145 mL of 0.212 M AgNO3(aq), both at 21.1°C, are mixed in a coffee cup calorimeter, the temperature of the mixture increases to 23.7°C as solid AgCl forms. NaCl(aq) + AgNO3(aq) → AgCl(s) + NaNO3(aq) This precipitation reaction produces 3.14 ✕ 103 J of heat, assuming no heat is absorbed by the calorimeter, no heat is exchanged between the calorimeter and its surroundings, and that the specific heat and density of the solutions are the same as those for water (4.18 J/g·°C, and 0.997 g/mL, respectively). Using this data, calculate ΔH in kJ/mol of AgNO3(aq) for the given reaction.arrow_forwardThe reaction shown below represents the formation reaction of water. 2 H2 (g) + O2 (g) → 2 H2O (g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY