
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
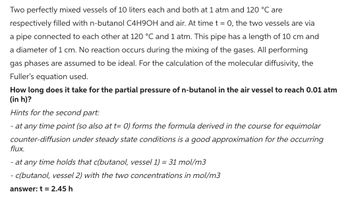
Transcribed Image Text:Two perfectly mixed vessels of 10 liters each and both at 1 atm and 120 °C are
respectively filled with n-butanol C4H9OH and air. At time t = 0, the two vessels are via
a pipe connected to each other at 120 °C and 1 atm. This pipe has a length of 10 cm and
a diameter of 1 cm. No reaction occurs during the mixing of the gases. All performing
gas phases are assumed to be ideal. For the calculation of the molecular diffusivity, the
Fuller's equation used.
How long does it take for the partial pressure of n-butanol in the air vessel to reach 0.01 atm
(in h)?
Hints for the second part:
- at any time point (so also at t= 0) forms the formula derived in the course for equimolar
counter-diffusion under steady state conditions is a good approximation for the occurring
flux.
- at any time holds that c(butanol, vessel 1) = 31 mol/m3
- c(butanol, vessel 2) with the two concentrations in mol/m3
answer: t = 2.45 h
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 10 images

Knowledge Booster
Similar questions
- Consider the reaction below to be at equilibrium: 4 A(e) + 3 B(e) + heat 5 Ce) + 3 D(e) In which direction will the reaction shift if more D is added? O It will not shift O It will shift toward the products. O It will shift toward the reactants.arrow_forwardTry I need itarrow_forwardA separation stream off the main reactor effluentcontains almost exclusively ethyl benzene,benzene, and toluene at 1 bar and 100°C. You determinethat the stream flow rate is made up of34 kg/s of benzene, 10 kg/s of toluene, and 57.75kg/s of the other component. You send this mixtureinto a flash distillation unit operating at 0.6bar and 100°C.A. Estimate if this mixture flashes.B. If the mixture flashes, determine the compositionand amount of the equilibrium liquid andvapor.C. You send the liquid exiting the flash distillationunit into another flash distillation unitoperating at 1.5 bar and 140°C. Determineif this mixture flashes. If so, determine thecomposition and amounts of the equilibriumphases.D. What percentage of the original benzene thatleft the reactor is now a vapor (you have to considerboth flash units).arrow_forward
- Consider a gaseous phase reaction X+Y →2Z that is happening in a reactor vessel. The reactants are at conditions of 298 K and 2 atm. Calculate the outlet concentration of Y, if the inlet concentration of X and Y are 4 mollit and 3 mol/lit respectively. The reaction is operating at 3 atm and 320 K. outlet concentration of X is 2 mollit. a) 2.39 mol/lit b) 1.39 mol/lit c) 3.39 mol/lit d) 2.39 mol/marrow_forwardSolve this question in a way that differs from the method solved on the website, in less than 30 minutesarrow_forwardWrite out the gibbs phase rule equation & calculate the degrees of freedom. Air (02 & N2) w/ water vapor, in equilibrium w/ a container that holds liquid water and solid water (Ice) & no reactions Ethanol & acetic acid to form ethyl acetate & water. Reaction occurs in the liquid phase, but both liquid & vapor are present.arrow_forward
- Please refer to the Chemical engineering handbook in determining the constants and show me the step-bystep solution in order for me to understand the solution clearly. Thank you.arrow_forwardButane (CH) is burned with 25% exceed air during a combustion process. Assuming complete combustion, determine the following for this reaction: a) air-fuel ratio, fuel-air ratio. equivalence ratio and Can part of the fuel not be burned? Show the details of your solution, including the balances of the actual and ideal reactionsarrow_forwardAn azeotropic mixture of ethanol and water is to be separated in a distillation column using benzene as an entrainer. At the column operating conditions, two liquid phases are formed on a tray. The degree (s) of freedom of the system for the choice of intensive properties at equilibrium is (are)arrow_forward
- 5. Hydrogen H2 is burned completely with the stoichiometric amount of air during a steady state, stead flow combustion process. If both reactants and products are maintained at 25 C and 1 atm, and the water in the products exists in the liquid form, determine the heat transfer from the combustion chamber during the process. What would your answer be if the combustion were achieved with 80% excess air ? Ans. (a) Q = 285,830 ku/kmol H2, (b) challenge *6 at 25 Larrow_forwardThe following gas-phase reaction takes place in a closed, constant-volume batch reactor at isothermal (constant temperature) conditions. 2CO+O2→2CO2 Initially, the reactor contains 0.564 kmol of CO, 1 kmol of O2, 1 kmol of CO2, and 0.5 kmol of N2 at a total pressure of 5 atm. At time t, the reactor pressure is 4.5 atm. Assuming ideal gas behavior, what is the extent of reaction (in kmol) at time t?arrow_forwarda) A chemically reacting mixture is stored in a thin-walled spherical metal container (inner radius r1 and outer radius r2) and the exothermic reaction generates heat at a spatially uniform, but temperature dependent volumetric rate of ġ = ġo-4/To. Verify that the steady solution to this problem for the spherical shell is given by 1 T(r) = Ts,1 – (T51-Ts,2) 1 ri Sketch the temperature distribution, labelling key features (temperatures, radii) b) Applying Fourier's law, determine rate of heat conducted through the spherical container. Also determine the heat flux for the two edges of the shells.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The