
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:Two nested spherical tanks with the internal and outer
diameters of the 100 cm by 104 cm and 114 cm by 118 cm is
used to store hot water at 100 C. Both tanks are made of boron
fiber epoxy with different composite compositions. The
thermal conductivity of the inner tank is 1.5 W/m K while the
outer tank has a thermal conductivity of 0.5 W/m K. The gap
between the tanks is filled with air (use properties of air at
50°C). The tank is located in an open environment at 0'C. The
outer surface of the tank is white painted and heat transfer
between the outer surface of the tank and the surrounding is
by natural covection and radiation. The convection heat
transfer coefficient at the inner and the outer surface of the
pipe is h 20 W/m' K and h 10 W/m K. Determine;
a. the rate of heat loss from the tank
b. the inside, outside and intermediate surface temperatures.
Hint: Take the outer surface temperature as 3°C for radiation
calculations.
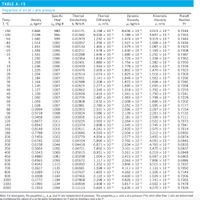
Transcribed Image Text:TABLE A-15
Properties of air at 1 atm pressure
Dynamic
Viscosity
µ, kg/m-s
Kinematic
Prandtl
Specific
Heat
Thermal
Thermal
Temp.
T, °C
Conductivity
k, W/m-K
Density
Diffusivity
Viscosity
v, m?/s
Number
P, kg/m³
Cp, J/kg-K
a, m?/s
Pr
-150
-100
8.636 x 10-6
1.189 x 10-5
2.866
983
0.01171
4.158 x 10-6
3.013 x 10-6
0.7246
8.036 x 10-6
1.252 x 10-5
1.356 x 10-5
1.465 x 10-5
1.578 x 10-5
1.696 x 10-5
2.038
966
0.01582
5.837 x 10-6
0.7263
0.01979
0.02057
-50
1.582
999
1.474 x 10-5
9.319 x 10-6
0.7440
1.527 x 10-5
1.579 x 10-5
1.630 x 10-5
1.680 x 10-5
1.729 x 10-5
1.754 x 10-5
1.778 x 10-5
1.802 x 10-5
1.825 x 10-5
1.849 x 10-5
1.872 x 10-5
-40
-30
1.514
1.451
1002
1004
1.008 x 10-5
1.087 x 10-5
0.7436
0.7425
0.7408
0.02134
0.02211
0.02288
-20
1.394
1005
1.169 x 10-5
-10
1.341
1006
1.252 x 10-5
0.7387
1.292
1006
0.02364
1.818 x 10-5
1.338 x 10-5
0.7362
1.880 x 10-5
1.944 x 10-5
2.009 x 10-5
2.074 x 10-5
2.141 x 10-5
2.208 x 10-5
1.269
1006
0.02401
1.382 x 10-5
0.7350
1.246
1.225
10
1006
0.02439
1.426 x 10-5
0.7336
15
1007
0.02476
1.470 x 10-5
0.7323
1007
1007
1007
1.516 x 10-5
1.562 x 10-5
1.608 x 10-5
1.655 x 10-5
20
1.204
0.02514
0.7309
25
1.184
0.02551
0.7296
30
1.164
0.02588
0.7282
35
1.145
1007
0.02625
2.277 x 10-5
1.895 x 10-5
0.7268
2.346 x 10-5
2.416 x 10-5
2.487 x 10-5
2.632 x 10-5
2.780 x 10-5
2.931 x 10-5
1.702 x 10-5
1.750 x 10-5
1.798 x 10-5
40
1.127
1007
0.02662
1.918 x 10-5
0.7255
1.109
0.02699
1.941 x 10-5
1.963 x 10-5
45
1007
1007
0.7241
50
1.092
0.02735
0.7228
0.02808
0.02881
2.008 x 10-5
2.052 x 10-5
2.096 x 10-5
1.896 x 10-5
1.995 x 10-5
2.097 x 10-5
2.201 x 10-5
60
1.059
1007
0.7202
1.028
0.9994
70
1007
0.7177
0.02953
0.7154
0.7132
80
1008
1008
3.086 x 10-5
3.243 x 10-5
3.565 x 10-5
2.139 x 10-5
2.181 x 10-5
2.264 x 10-5
2.345 x 10-5
90
0.9718
0.03024
100
0.9458
1009
0.03095
2.306 x 10-5
0.7111
120
0.7073
0.7041
0.8977
1011
0.03235
2.522 x 10-5
0.8542
0.8148
2.745 x 10-5
2.975 x 10-5
140
1013
0.03374
3.898 x 10-5
4.241 x 10-5
4.593 x 10-5
4.954 x 10-5
5.890 x 10-5
6.871 x 10-5
7.892 x 10-5
2.420 x 10-5
2.504 x 10-5
2.577 x 10-5
2.760 x 10-5
2.934 x 10-5
160
1016
0.03511
0.7014
180
0.7788
1019
1023
0.03646
3.212 x 10-5
0.6992
200
0.7459
0.03779
3.455 x 10-5
0.6974
0.6746
250
300
1033
0.04104
4.091 x 10-5
0.6946
4.765 x 10-5
5.475 x 10-5
0.6158
1044
0.04418
0.6935
3.101 x 10-5
3.261 x 10-5
3.415 x 10-5
3.563 x 10-5
3.846 x 10-5
350
0.5664
1056
0.04721
0.6937
6.219 x 10-5
6.997 x 10-5
7.806 x 10-5
9.515 x 10-5
8.951 x 10-5
1.004 x 10-4
1.117 x 10-4
1.352 x 10-4
1.598 x 10-4
1.855 x 10-4
2.122 x 10-4
2.398 x 10-4
3.908 x 10-4
5.664 x 10-4
0.6948
0.6965
400
0.5243
1069
0.05015
450
0.4880
1081
0.05298
500
0.4565
1093
0.05572
0.6986
600
0.4042
1115
0.06093
0.7037
4.111 x 10-5
4.362 x 10-5
700
0.3627
1135
0.06581
1.133 x 10-4
0.7092
1.326 x 10-4
1.529 x 10-4
800
0.3289
1153
0.07037
0.7149
900
0.3008
1169
0.07465
4.600 x 10-5
0.7206
1000
1500
4.826 x 10-5
5.817 x 10-5
6.630 x 10-5
0.2772
1184
0.07868
1.741 x 10-4
0.7260
0.09599
2.922 x 10-4
4.270 x 10-4
0.1990
1234
0.7478
2000
0.1553
1264
0.11113
0.7539
Note: For ideal gases, the properties C, k, µ, and Pr are independent of pressure. The properties p, v, and a at a pressure P (in atm) other than 1 atm are determined
by multiplying the values of p at the given temperature by Pand by dividing v and a by P.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A supply of 50 kg of chicken at 6degC contained in a box is to be frozen to -18degC in a freezer. Determine the amount of heat that needs to be removed. The latent heat of chicken is 247 kJ/kg and its specific heat is 3.32 kJ/kg-degC above freezing and 1.77 kJ/kg-degC below freezing. The freezing temperature of the chicken is -2.8degCarrow_forwardConsider a furnace (as a plane wall) which has a thickness of 15 cm and a surface area of 1 m?. The inside surface of the furnace wall (the hot left side of the wall) is at temperature T1. The furnace wall is made of brick with thermal conductivity of 1.198 W/m.K. The outside surface of the furnace wall (the right side of the wall) has a surface emissivity of 0.8 and is maintained at T2=101 °C and subject to air at 25 °C with convection heat transfer coefficient of 20 W/m?.K. The surroundings temperature at the outside of the furnace wall is at 25 °C. Required: Draw a clear and consistent schematic of the problem and label the operating conditions on the schematic. Perform systematic analysis, state your assumptions and justify the equation used and determine the following (circle your final answers): (i) The rate of heat transfer by convection (in W); (ii) The rate of heat radiation (in W); (iii) The surface temperature of the furnace wall T1 (in °C); (iv) The rate of conduction heat…arrow_forwardConsider a double-paned window consisting of two panes of glass, each with a thickness of 0.500 cm and an area of 0.795 m2 , separated by a layer of air with a thickness of 1.50 cm. The temperature on one side of the window is 0.00 ∘C∘C; the temperature on the other side is 21.0 ∘C∘C. In addition, note that the thermal conductivity of glass is roughly 36 times greater than that of air. Approximate the heat transfer through this window by ignoring the glass. That is, calculate the heat flow per second through 1.50 cmcm of air with a temperature difference of 21.0 ∘C∘C. (The exact result for the complete window is 25.6 J/sJ/s .)arrow_forward
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY