
Concept explainers
Two identical metal blocks of mass 20 kg and specific heat 0.4 kJ/kg are at an initial temperature of 50 degree Celsius .A reversible refrigerator receives heat from one block and rejects heat to the other. Find the work required by the refrigerator to cause a temperature difference of 150 degree Celsius between the two blocks.
Let us call the two blocks as block1 and block2. (Refer to Figure1)
Mass of each block (m)= 20kg.
Specific heat of each block (c)=0.4 kJ/kg.
Initial temperature of both the blocks is same (Ti)= 500C=323K.
Final Temperature of block1=Tf1.
Final Temperature of block2=Tf2.
Since heat is absorbed from block2 and rejected to block1 ,Tf1 > Tf2 and it is given that Tf1 - Tf2 = 150.
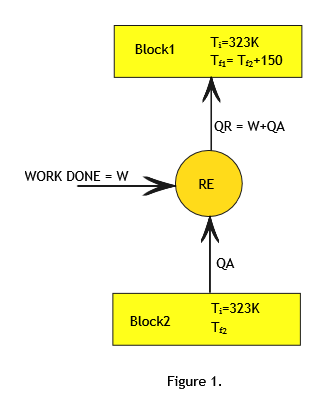
Since the refrigerator is reversible refrigerator all the processes involved are reversible processes.
According to Principle of entropy, for reversible processes change in entropy of the Universe is zero.

Calculate change in entropy of body1 and body2 as follows:
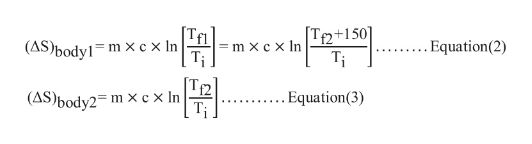
Substitute Equations (2) and (3) in Equation (1)
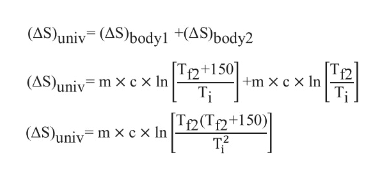
Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 5 images

- For irreversible adiabatic expansion or compression of an ideal gas, work is equal to the internal energy change. Select one: True Falsearrow_forwarda) A gas is trapped in a piston-cylinder device. The initial pressure and temperature of the gas are 500 kPa and 300 K, respectively. The system undergoes an isothermal expansion process, in which 15KJ of boundary work is done by the gas and 3KJ of paddle-wheel work is done on the gas. Determine the amount heat transfer during this process. Explain the direction of the heat transfer.arrow_forwardA copper container of mass 0.080 kg and specific heat 387 Jkg-1 K-1 contains 0.30 kg of water and 0.040 kg of ice at 0˚C. Steam at 100˚C is passed into the water and its temperature stabilizes at 20.0˚C. Find the mass of the water left in the container. Assume the system is insulated from its environment.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





