
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
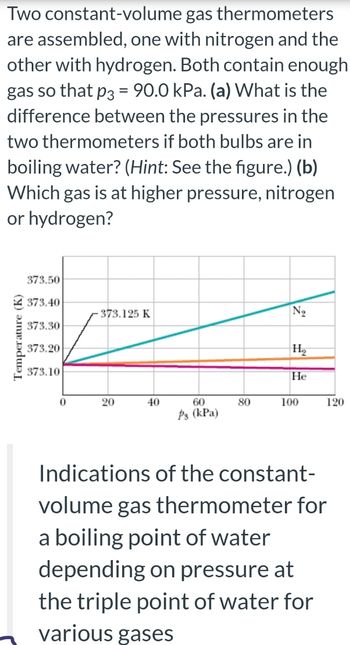
Transcribed Image Text:Two constant-volume
gas thermometers
are assembled, one with nitrogen and the
other with hydrogen. Both contain enough.
gas so that p3 = 90.0 kPa. (a) What is the
difference between the pressures in the
two thermometers if both bulbs are in
boiling water? (Hint: See the figure.) (b)
Which gas is at higher pressure, nitrogen
or hydrogen?
373.50
373.40
373.30
373.20
-373.125 K
Z
373.10
0
20
40
60
P3 (kPa)
80
N₂
H₂
He
100
120
Indications of the constant-
volume gas thermometer for
a boiling point of water
depending on pressure at
the triple point of water for
various gases
Transcribed Image Text:(a) Number
(b)
i
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 5E. When a mercury-in-glass thermometer is overheated, the top will break off due to the pressure increase from the expansion of mercury which has completed filled the volume of the capillary. A thick, well-tempered glass tube may withstand a maximum pressure of 50 atm without breaking. How far can a thermometer be heated past the temperature at which a capillary is filled before the pressure becomes this large? For mercury, a=1.8x10ª K' and K=3.9x106 atm"'arrow_forward1. An insulated cylinder contains an ideal gas. The initial volume, pressure and temperature of the gas are 3.20 x 104 m², 1.01 x 10° Pa and 320 K respectively. Assume that there is no heat transferred through the piston. a) Calculate the number of moles of the gas. [1.22 x 10* mol] b) The gas is compressed until its volume becomes 4.50 x 10-5 m³ and the temperature becomes 750 K. Calculate the final pressure of the gas. [1.69 x 10 Pa] c) The work done on the gas is 120 J. Calculate the increase in the internal energy of the gas. [120 J]arrow_forwardAn electric motor has an effective resistance of 68 and an inductive reactance of 85 when working under load. The voltage amplitude across the alternating source is 420V. Calculate the current amplitude in A.arrow_forward
- The p-V diagram in the figure shows two paths along which a sample of gas can be taken from state a to state b, where V = 3.0V1. Path 1 requires that energy equal to 5.0p,V be transferred to the gas as heat. Path 2 requires that energy equal to 5.5p,V, be transferred to the gas as heat. Determine the ratio 2. P1 P2 2 Varrow_forwardA tank contains one mole of nitrogen gas at a pressure of 5.20 atm and a temperature of 24.5°C. The tank (which has a fixed volume) is heated until the pressure inside triples. What is the final temperature of the gas? °C (b)A cylinder with a moveable piston contains one mole of nitrogen, again at a pressure of 5.20 atm and a temperature of 24.5°C. Now, the cylinder is heated so that both the pressure inside and the volume of the cylinder double. What is the final temperature of the gas? °Carrow_forwardAn ideal gas at 17.1 °C and a pressure of 1.96 x 10 Pa occupies a volume of 3.21 m. (a) How many moles of gas are present? (b) If the volume is raised to 4.05 m and the temperature raised to 25.0 °C, what will be the pressure of the gas? (a) Number Units (b) Number Unitsarrow_forward
- A sealed 73 m tank is filled with 8000 moles of ideal oxygen gas (diatomic) at an initial temperature of 270 K. The gas is heated to a final temperature of 390 K. The atomic mass of oxygen is 16.0 g/mol. The mass density of the oxygen gas, in Sl units, is closest to: O 7.0 O 4.4 O 2.6 О 35 O 1.8arrow_forwardA gas thermometer of constant volume is filled to a pressure P3 in equilibrium with the triple point of water. Then it is placed in thermal equilibrium with a system of unknown temperature at a pressure P. This is repeated five times, the data obtained are: P3 P 500 882 400 707.4 300 527.4 200 351.0 What is the temperature of the idea gas of the system? Remember: T3=273.16 100 175.2arrow_forwardThe gas inside a balloon will always have a pressure nearly equal to atmospheric pressure since that is the pressure applied to the outside of the balloon. You fill a balloon with helium (a nearly ideal gas) to a volume of 0.560 L at a temperature of 17.5 ∘C. What is the volume of the balloon if you cool it to the boiling point of liquid nitrogen, 77.3 K?arrow_forward
- Two containers of equal volume each hold samples of the same ideal gas. Container A has twice as many molecules as container B. If the gas pressure is the same in the two containers, the correct statement regarding the absolute temperatures TA and TB in containers A and B, respectively, is TA = TB TA = 2TB %3D TA = TB/2 TA= 1B//2 TA= TB/4 %3Darrow_forwardTwo small containers, each with a volume of 100 cm3, contain helium gas at 0°C and 1.00 atm pressure. The two containers are joined by a small open tube of negligible volume, allowing gas to flow from one container to the other. What common pressure will exist in the two containers if the temperature of one container is raised to 100°C while the other container is kept at 0°C?arrow_forwardFor many purposes we can treat propane (C3H8) as an ideal gas at temperatures above its boiling point of −42.°C. Suppose the temperature of a sample of propane gas is lowered from 60.0°C to 10.0°C, and at the same time the pressure is increased by 15.0%. Does the volume of the sample increase, decrease, or stay the same? increase decrease stays the same If you said the volume increases or decreases, calculate the percentage change in the volume. Round your answer to the nearest percent. %arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON