
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
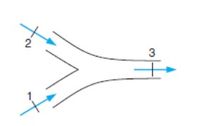
Two air flows are combined to a single flow. One flow is 1 m3/s at
20oC and the other is 2 m3/s at 200oC, both at 100 kPa. They mix
without any heat transfer to produce an exit flow at 100 kPa. Neglect kinetic energies and find the exit temperature and volume flow rate.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill up the two missing values inside the two circles knowing that the total reaction volume is 2mL. 1) Dilution fraction 2) Water Don't include units in your answer. Membrane Brillant blueR 1g/L Dilution Sample suspension fraction (μL) (μL) 1) Control ? 0 50 2) 1/200 ? 10 50 3) 1/100 0.01 20 50 4) 1/50 ? 40 50 5) 1/20 100 50 Phosphate pH11, 0.2M (ml) 1 1 1 1 1 Water (mL) 0.95 ? ? ?arrow_forwardCalculate the equilibrium constant (K'eg) for each of the three reactions at pH 7.0 and 25 °C, using the AGʻ° values given. (a) glucose 6-phosphatase glucose 6-phosphate + H,O glucose + P; AG'° = –13.8 kJ/mol K'eq= (b) B-galactosidase lactose + H,O glucose + galactose AG'O = -15.9 kJ/mol K'eq=arrow_forwardCount the significant digits in each of these measurements: number of measurement significant digits ? 82300. kg 0.005100 J - 3.0 x 10 -2 kJ/mol -1 3.1 x 10 mLarrow_forward
- Following several days at high altitude the body responds to the decreased oxygen content of the atmosphere by increasing the amount of 2,3-bisphosphoglycerate it produces. Based on this information and refering to the graph below, identify the correct response from the options. Y (fraction saturation) 1.0 0.8 0.6 0.4 0.2 0.0 0 20 Oxygen Binding plot 40 60 p02 (torr) 80 100 A) Curve y=high altitude and gives a greater release of oxygen to the tissues B) Curve x = high altitude and gives a greater release of oxygen to the tissues C) Curve y=high altitude and allows more oxygen to be bound at low oxygen levels D) Curve x = high altitude and allows more oxygen to be bound at low levelsarrow_forwardThe kinetic data in the following table were obtained for the reaction of carbon dioxide and water to produce bicarbonate and hydrogen ion catalyzed by carbonic anhydrase. H2O + CO2--> HCO3 + H+ Carbon Dioxide Concentration (mmol L-') 1.25 2.5 5.0 20.0 1/Velocity (M'sec) 36 x 10³ 20 x 10³ 12 x 10³ 6 x 10³ [H. DeVoe and G. B. Kistiakowsky, J. Am. Chem. Soc. 1961 83 (2), 274-280.] Use Excel (or similar) to generate a Line Weaver-Burke plot, then use the equation for a linear-fitted trend line to calculate the substrate affinity of CO2 for carbonic anhydrase. Answer in units of mM. Enter numerical values only, no letters or symbols. Round answer to 2 significant figures.arrow_forwardCalculate for rate constant(k)arrow_forward
- Example The standard half electrode potential for Fe²+ vs SHE is 0.77 V. What is the value of Ee at T = 298K when: [Fe³+] = 0.2 mol l-1 [Fe2+] = 0.05 mol 1-1arrow_forwardDuring transfer of liquids using a micropipette: What would it mean if the mean is very close to the expected value but your standard deviation is high ?arrow_forwardWhat is the expected condition for a patient with following ABG values of pH 7.5, pco2-46mmHg, HCO--30 m Eq/dL and k+-3.4 mEq/dL Metabolic alkolosis Metabolic acidosis Respiratory alkolosis Respiratory acidosisarrow_forward
- Using Hb + O2 <-> HbO2, show how the equation shifts according to the law of mass action when O2 concentration increases or decreases.arrow_forwardPlease look at image for question! Please help.arrow_forwardWhat term describes kcat/Km? What is the maximum value that this term can attain? Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON