
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
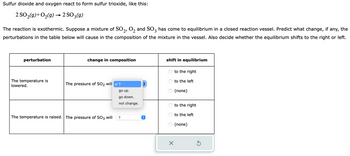
Transcribed Image Text:Sulfur dioxide and oxygen react to form sulfur trioxide, like this:
2 SO₂(g) + O₂(g) → 2 SO 3(g)
The reaction is exothermic. Suppose a mixture of SO2, O₂ and SO3 has come to equilibrium in a closed reaction vessel. Predict what change, if any, the
perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left.
perturbation
The temperature is
lowered.
change in composition
The pressure of SO₂ will ✓?
The temperature is raised. The pressure of SO3 will
go up.
go down.
not change.
?
↑
shift in equilibrium
OO
OOO
X
to the right
to the left
(none)
to the right
to the left
(none)
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question

Transcribed Image Text:Try Again
Your answer is incorrect.
• Row 1: Your answer for change in composition column is incorrect.
• Row 2: Your answer for change in composition column is incorrect.
Sulfur dioxide and oxygen react to form sulfur trioxide, like this:
2 SO₂(g) + O₂(g) → 2 SO 3(g)
The reaction is exothermic. Suppose a mixture of SO₂, O₂ and SO3 has come to equilibrium in a closed reaction vessel. Predict what change, if any, the
perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left.
perturbation
The temperature is
lowered.
change in composition
The pressure of SO₂ will
The temperature is raised. The pressure of SO3 will
go up.
go up.
↑
shift in equilibrium
to the right
to the left
(none)
to the right
to the left
(none)
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question

Transcribed Image Text:Try Again
Your answer is incorrect.
• Row 1: Your answer for change in composition column is incorrect.
• Row 2: Your answer for change in composition column is incorrect.
Sulfur dioxide and oxygen react to form sulfur trioxide, like this:
2 SO₂(g) + O₂(g) → 2 SO 3(g)
The reaction is exothermic. Suppose a mixture of SO₂, O₂ and SO3 has come to equilibrium in a closed reaction vessel. Predict what change, if any, the
perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left.
perturbation
The temperature is
lowered.
change in composition
The pressure of SO₂ will
The temperature is raised. The pressure of SO3 will
go up.
go up.
↑
shift in equilibrium
to the right
to the left
(none)
to the right
to the left
(none)
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Sulfur dioxide and oxygen react to form sulfur trioxide, like this: 2 SO₂(g) + O₂(g) → 2 SO 3(g) The reaction is exothermic. Suppose a mixture of SO2, O₂ and SO3 has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation The temperature is lowered. change in composition The pressure of SO3 will The temperature is raised. The pressure of SO₂ will ? ? î ↑ shift in equilibrium O X to the right to the left (none) to the right to the left (none) Sarrow_forwardAmmonia and oxygen react to form nitrogen and water, like this: 4NH3(g)+30₂(g) → 2N₂(g) + 6H₂O(g) Suppose a mixture of NH3, O₂, N₂ and H₂O has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation Some NH3 is removed. Some N₂ is added. change in composition The pressure of O₂ will The pressure of N₂ will The pressure of NH3 will The pressure of O₂ will ? ? ? ? O O C C shift in equilibrium O to the right Oto the left O (none) X O to the right O to the left O (none) 5 E Carrow_forwardNitrogen and hydrogen react to form ammonia, like this: N,(9)+3H,(g) → 2 NH3(g) The reaction is exothermic. Suppose a mixture of N,, H, and NH, has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. olo perturbation change in composition shift in equilibrium Ar to the right The temperature is lowered. to the left The pressure of N2 will (none) to the right to the left The temperature is raised. The pressure of NH3 will v ? go up. (none) go down. not change. ?arrow_forward
- Ammonium carbamate, NH4CO₂NH₂, decomposes as follows: NH,CO,NH,(s)→ 2NH,(g) + CO, (g) Starting with only the solid, it is found that at 40.0 °C the total gas pressure (NH3 and CO₂) is 0.463 atm. Calculate the equilibrium constant Kp. Round your answer to 3 significant digits. 31 K₂ = 11 Kp x10 X Śarrow_forwardMethane and water react to form hydrogen and carbon monoxide, like this: CH₁(9)+H₂O(g) → 3H2(g)+CO(g) The reaction is endothermic. Suppose a mixture of CH 4, H2O, H2 and CO has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation change in composition shift in equilibrium to the right to the left The temperature is raised. The pressure of CH4 will ? ○ (none) to the right to the left The temperature is lowered. The pressure of CO will ? O (none) ×arrow_forwardSulfur dioxide and oxygen react to form sulfur trioxide, like this: 2 SO,(9)+O2(g) → 2 SO3(g) The reaction is exothermic. Suppose a mixture of SO2, O, and SO, has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. dlo perturbation change in composition shift in equilibrium Ar to the right The temperature is lowered. to the left The pressure of SO3 will ? (none) to the right to the left The temperature is raised. The pressure of SO2 will ? O (none)arrow_forward
- A chemical engineer is studying the following reaction: HCH3CO2(aq)+CH3NH2(aq) → CH3CO2(aq)+CH3NH(aq) At the temperature the engineer picks, the equilibrium constant K for this reaction is 0.80. The engineer charges ("fills") four reaction vessels with acetic acid and methylamine, and lets the reaction begin. She then measures the composition of the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time she measures the compositions. reaction vessel compound concentration expected change in concentration HCH CO₂ 0.58 M ↑ increase ↓ decrease (no change) CH3NH2 0.57 M ↑ increase ↓ decrease (no change) A CH3CO2 1.09 M ○ ↑ increase ↓ decrease (no change) CH,NH, 1.28 M ○ ↑ increase ↓ decrease (no change) HCH,CO₂ CH3NH2 1.28 M ↑ increase ↓decrease (no change) 1.27 M ↑ increase ↓ decrease (no change) B CH,CO, 0.39 M ↑ increase ↓ decrease (no change) CHÍNH, HCH3CO2…arrow_forwardSulfur dioxide and oxygen react to form sulfur trioxide, like this: 2 SO,(g)+O,(g) –→ 2 SO3(9) The reaction is exothermic. Suppose a mixture of SO,, O, and SO, has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation change in composition shift in equilibrium O to the right The temperature is raised. The pressure of SO3 will O to the left O (none) O to the right The temperature is lowered. The pressure of SO2 will v ? O to the left go up. O (none) go down. not change. Explanation Check 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Cent b國 TO00 FEB 21 23 tv MacBook Air DII DD 30 888 F7 F8 F9 10 esc F2 F4 F5 F6 F1 F3 #3 $ 6. 8 9 ....arrow_forwardWhat is the equilibrium concentration of BCl3 if a solid sample of PH3BC13 is placed in a 0.250 L closed vessel at 80.0 °C and decomposes until equilibrium is reached? Assume that there is solid present at equilibrium. At 80.0 °C, Kc = 1.87 x 10-³ for the reaction: PH3BC13(s) ⇒ PH3(g) + BCl3(g) Provide your answer in M, without units, and use the correct number of significant figures. 534.8arrow_forward
- please see attached imagearrow_forwardAssume that the reaction for the formation of gaseous hydrogen fluoride from gaseous hydrogen and gaseous fluorine has an equilibrium constant of 1.15 x 102 at a given temperature. In a particular experiment, 3.00 mol of each component was added to a 1.50 L flask. Calculate the equilibrium concentrations of all species.arrow_forwardAmmonia and oxygen react to form nitrogen and water, like this: 4NH, (g)+30,(g) 2N,(g)+6H,O(g) Suppose a mixture of NH2, O2, N, and H,0 has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation change in composition shift in equilibrium O to the right The pressure of NH3 will Some N, is added. O to the left The pressure of Oz will ? O (none) O to the right The pressure of O, will ? O to the left Some NH, is removed. The pressure of N2 will O (none) Submit Assignme Continue MacBook Air FI0 F8 吕口 D00 000 F4 F5 F3 F2 %arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY