
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
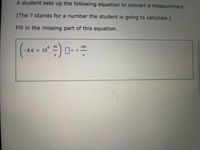
Needing help understanding this. I've been stuck and don't seem to understand at all please help.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rearrange equation 1 to solve for the molecular weight of a compound assuming you know the mass of solute addedarrow_forward/Explain how the two vitamins below reacts with water.Ensure you use the following polar,non-polar sigma and pi bonds. Identify all the functional groups present and describe how and why they interact with water. Vitamin C (Ascorbic acid) Vitamin E (alpha-topherol) HỌ HO. HO. OH HO H₂C H₂ CH₂ CH₂arrow_forwardPlease send me the question in 20 minutes it's very urgent plz question 21 Part 1 and 2arrow_forward
- Name 10 dissolved substances that negatively affect the environment.arrow_forwardRoom temperature: 295.9 K Barometric pressure: 769.0 mmHg Vapor of water: 21.1 mmHg Volume of O2 collected: 59.20 mL Density of H2O2: 1.01 g/mL % Composition H2O2: 3.02 % Volume of H2O2 used: 5.00 mL Letter of the unknown solution of H2O2: C Volume of O2 collected for the unknown: 64.90 mL Calculate R in the ideal gas law.arrow_forwardWhat volume of 12 M HCI is required to prepare 16 L of 0.25 M HCI? 250 mL 333 mL 768 mL 585 mL O 130 mLarrow_forward
- Room temperature: 294.0 K Barometric pressure: 770.5 mmHg Vapor of water: 18.6 mmHg Volume of O2 collected: 57.90 mL Density of H2O2: 1.01 g/mL % Composition H2O2: 3.02 % Volume of H2O2 used: 5.00 mL Letter of the unknown solution of H2O2: B Volume of O2 collected for the unknown: 20.20 mL corrected barometric pressure. 752 mmHg Expert, what is a term letter?arrow_forwardA student prepares a 0.29 M aqueous solution of propionic acid (C₂H,CO₂H). Calculate the fraction of propionic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. % Xarrow_forwardComplete and balance the following neutralization reactions. H2CO3(aq)+Ba(OH)2(aq)→H2CO3(aq)+Ba(OH)2(aq)→ Express your answer as a chemical equation. Identify all of the phases in your answer.arrow_forward
- In 1965, University of Florida assistant football coach be concerned with his players performance. They were "tapping out" around half-time/3rd quarter and were really struggling during summer "hell week" to make it through a day. very He turned to a panel of doctors to trywand figure out what he could do to help his players maintain their stamina in the Florida weather. Their solution... GATORADE! • Gatorade consists of sugar (glucose), sodium (Na), Potassium (K) and water. All three of these electrolytes were needed. 1. What pumps and transporters were these physicians targeting with their concoction? 2. Why did they need ALL THREE solutes to benefit the players?arrow_forward1. Water is necessary for life on Earth. Water is made up of hydrogen and oxygen atoms (H2O); however, biological life in water depends on another form of oxygen. These organisms need molecular oxygen (O2), which they use to perform aerobic respiration. This molecular oxygen fits into spaces between water molecules and is available for aquatic organisms to use. How do you think molecular oxygen gets into the water? Choose two of the following choices. Select all that apply: From the oxygen atoms that are part of the water molecules. From oxygen that dissolves into water from the atmosphere. From oxygen that is given off by aquatic organisms during respiration. From oxygen that is given off by aquatic plants during photosynthesis.arrow_forwardHey can you help me answer this question? Thank youarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY