
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Oo.53.
Subject:- Chemistry
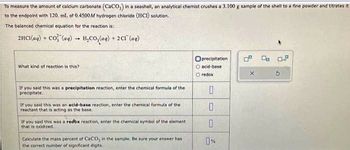
Transcribed Image Text:To measure the amount of calcium carbonate (CaCO3) in a seashell, an analytical chemist crushes a 3.100 g sample of the shell to a fine powder and titrates it
to the endpoint with 120. ml. of 0.4500M hydrogen chloride (HCI) solution.
The balanced chemical equation for the reaction is:
2HCl(aq) +
Cơ, lạng)
-
What kind of reaction is this?
H₂CO3(aq) + 2Cl (aq)
If you said this was a precipitation reaction, enter the chemical formula of the
precipitate.
If you said this was an acid-base reaction, enter the chemical formula of the
reactant that is acting as the base.
If you said this was a redox reaction, enter the chemical symbol of the element
that is oxidized.
Calculate the mass percent of CaCO, in the sample. Be sure your answer has
the correct number of significant digits.
Oprecipitation
O acid-base
O redox
0
0
0
0%
9.
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following reactions:CoO (s) + CO (g) D CO2 (g) + Co (s) Kc(1) = 490.2 CoO (s) + 2 H2 (g) D 2 Co (s) + 2 H2O (g) Kc(2) = 4.5 x 103a. Write the overall equation for the reaction of hydrogen gas and carbon dioxide gas to produce carbon monoxide gas and steam.arrow_forwardA Chemistry 20 student uses a thermometer and a hot plate and measures the boiling point of ethyl alcohol to be 74.3 ºC. Then, she looks in a reference book and finds that the actual boiling point of ethyl alcohol is 78.4 ºC. What is her percent error?arrow_forwardFor eac te, click the button under the better solvent. solute Which is the better solvent? :0: || :0: :0: CH,-S CH, НО -С — СH, — CH, — С — ОН CH;CH,OH CH; H C Harrow_forward
- Caps Tab Wall wen M Inbox (1.600)-ftantiudeledux Mail-Francesca A Tantillo-Out xEXP #12: Geometry-CHM150-2 x Aktiv Chemistry ← → C app.101edu.co 90°F Mostly sunny 1 D Q Z Uranium hexafluoride, UF, is an important compound used in the enrichment of uranium by gaseous diffusion. A A Graham's Law states that rate of effusion A/rate of effusion of B = (square root of Mass of B)/(square root of Mass of A). Calculate how fast 235 UF gas diffuses compared to 230UF. gas. Assume the temperature remains constant. 2 W S 3 E D # C $ 4 R OL F C ▷ll s FS % 5 T V C Question 19.c of 23 G P A 6 Y B H & 7 PrtScn N J 8 N Home 1 M ( 9 K End o O < 1 4 7 +/- PgUp 0 L times faster than 238UF 2 5 8 12 4 P 3 6 9 0 □ A PED PgOn 1924 G + 0 Update Submit C x 100 BE 5:22 PM 7/6/2022 Del Backspace 과arrow_forwardThe great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. 2. Suppose 71.0 mL of dioxygen gas are produced by this reaction, at a temperature of 50.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide that must have reacted. Be sure your answer has the correct number of significant digits.arrow_forward요 Eto OEt 1. EtMgBr(xs) 2. H3O* HOarrow_forward
- 2 Automobile air bags inflate following a serious impact. The impact triggers the following chemical reaction. 2NaN3 (8)→ 2 Na(s) + 3N₂ (9) S W stry.com/myct/itemView?assignmentProblemID=213377858&attemptNo=2&offset=next X 7 # 3 E D 80 C $ 4 R 888 F V % 5 FS T Y G A Part A 6 B If an automobile air bag has a volume of 11.2 L, what mass of NaN3 (in g) is required to fully inflate the air bag upon impact? Assume STP conditions. IVE ΑΣΦ Provide Feedback m = Submit MacBook Air F6 Y H & 7 Request Answer N 40 U J * 8 @ C PwC FB I M ( 9 K DD ? O ) O g L command 3 F10 P . : ; Review I Constants I Periodic Table I { + [ option = 11 ? 1 I 323) Next > 1 deletearrow_forwardPart C F10 BO O 3.04 × 10³ 6.27 x 10-1 The dosage of quinine when a 145-lb adult takes a 200.-mg tablet is equivalent to ug drug per kg of body weight. ► View Available Hint(s) O 627 O 1.38 × 10³ P Pearson * Education Inc. All rights reserved. | Terms of Use Privacy Policy. Permissions Contact Us | Bb Home L End Insert ■ Review |- Constants | Periodic Table 1 2arrow_forwardArchimedes tells us the lifting power of a balloon (how much mass it can lift) is equal to the difference between the mass of the balloon and the mass of the air it displaces. That is, if the balloon occupies the same volume as 10 kg of air, but the balloon only weighs 2 kg, then the gas density kg 0.090 3 m H, balloon can lift 8 kg. Airships have sometimes been filled with hydrogen (H,), but hydrogen is very flammable, and kg 0.18 Не m after the Hindenburg caught fire and crashed in 1937 with serious loss of life airships have nearly always been filled with helium (He) instead. kg 1.23 3 m' air Suppose a new airship Star of Balogna will have the shape of a cylinder 100. m long, with a radius of 15.0 m, and will weigh 9400. kg without any gas inside it. Suppose also the average passenger weighs 81 kg. Calculate how many passengers the Balogna could carry if it were filled with hydrogen, and also if it were filled with helium. Passengers with H,: Passengers with He:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY