
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Jset2_5zKMTIDEhEBBQc5Ge4YwKAkK5h...
O CHEMICAL REACTIONS
Solving for a reactant using a chemical equation
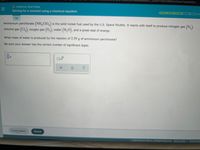
Ammonium perchlorate (NH CIO,) is the solid rocket fuel used by the U.S. Space Shuttle. It reacts with itself to produce nitrogen gas (N,),
chlorine gas (Cl,), oxygen gas (0,), water
(H,O), and a great deal of energy.
What mass of water is produced by the reaction of 2.59 g of ammonium perchlorate?
Be sure your answer has the correct number of significant digits.
Explnation
Check
2022 McGraw Hill LLC All Righnts Reserved
Terms of Use
Privacy Cen
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the number of hydrogen atoms in a 150.0 g sample of hydrazine (N,H). Be sure your answer has a unit symbol if necessary, and round it to 4 significant digits.arrow_forwardGaseous butane (CH, (CH. CH, reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). What is the theoretical yield of carbon dioxide formed from the reaction of 18.6 g of butane and 47.3 g of oxygen gas? Round your answer to 3 significant figures.arrow_forwardAmmonium phosphate PO4) NH,),PO.) РО 3 is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO) with ammonia (NH,). What mass of ammonium phosphate is produced by the reaction of 9.6 g of phosphoric acid? Round your answer to 2 significant digits.arrow_forward
- Gaseous ethane (CH,CH,) reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). What is the theoretical yield of carbon dioxide formed from the reaction of 24.1 g of ethane and 45.7 g of oxygen gas? Be sure your answer has the correct number of significant digits in it.arrow_forwardPlease don't provide handwritten solution .....arrow_forwardround it to the correct number of significant digitsarrow_forward
- Please put the correct number of significant figuresarrow_forwardM Use the References to access important values if needed for this question. For the following reaction, 5.08 grams of benzene (C6H6) are allowed to react with 19.7 grams of oxygen gas. benzene (C6H6) (1) + oxygen (g) → carbon dioxide(g) + water (g) What is the maximum amount of carbon dioxide that can be formed? Mass = 17.16 g What is the FORMULA for the limiting reactant? What amount of the excess reactant remains after the reaction is complete? Mass = 4.08 Submit Answer 6.0 g Retry Entire Group 8 more group attempts remaining Show Hint Previous Next> Save and Exiarrow_forwardGreen plants use light from the Sun to drive photosynthesis, a chemical reaction in which liquid water and carbon dioxide gas form aqueous glucose (C6H12O6) and oxygen (O2) gas. Calculate the moles of oxygen produced by the reaction of 2.20 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits.arrow_forward
- www-awn.aleks.com CHEMICAL REACTIONS Brittney V Solving for a reactant using a chemical equation A major component of gasoline is octane (CH8). When octane is burned in air, it chemically reacts with oxygen gas (0,) to produce carbon dioxide (CO,) and water (H,0). What mass of water is produced by the reaction of 3.03 g of octane? Round your answer to 3 significant digits. g Ar x10 Explanation Check © 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy | Accessibilityarrow_forwardGaseous methane (CH4) reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). What is the theoretical yield of water formed from the reaction of 0.48 g of methane and 1.6 g of oxygen gas? Be sure your answer has the correct number of significant digits in it.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY