
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
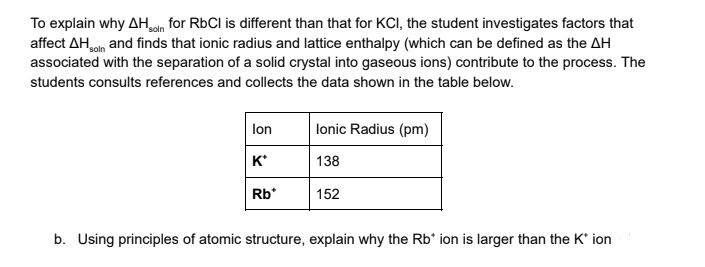
Transcribed Image Text:To explain why AHan for RbCl is different than that for KCI, the student investigates factors that
affect AHoln and finds that ionic radius and lattice enthalpy (which can be defined as the AH
associated with the separation of a solid crystal into gaseous ions) contribute to the process. The
students consults references and collects the data shown in the table below.
lonic Radius (pm)
lon
к'
138
Rb*
152
b. Using principles of atomic structure, explain why the Rb' ion is larger than the K' ion
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- For coordinate covalent bonds: What is a coordinate covalent bond? Use Lewis structures to show how H+ and NH3 form a coordinate covalent bond resulting in NH4+ (i.e. write out the reaction using Lewis structures). Why is it tough to recognize the coordinate covalent bond in NH4+? What is one way to recognize that something has a coordinate covalent compound? Why are they so important?arrow_forwardThe compound boron nitride (BN) is a compound semiconductor having mixed ionic and covalent bonding. The electronegativities for B and N are 2.0 and 3.0 respectively. Calculate the fraction of the bonding that is ionic. (Enter your answer to three significant figures.) fionicarrow_forwardUse the Born Haber cycle (show relevant steps) to determine the lattice energy of CsCl (s) from the following data: ΔHf°[CsCl(s)] = -442.8 kJ/mol; enthalpy of sublimation of Cesium is 78.2 kJ/mol; enthalpy of dissociation of Cl2 (g) = 243 kJ/mol Cl2 ; IE1 for Cs(g) = 375.7 kJ/mol; electron affinityenthalpy-EA1 for Cl(g) = -349kJ/mol.arrow_forward
- The energy for the following reaction was measured to be -653.0 kJ/mol. K(g) + Cl(g) →→KCI(s). Using this fact and the data in the table below, calculate the enthalpy (in kJ/mol) required to separate the ions from the lattice for this reaction: KCI(s) → K+(g) + Cl-(g). Substance K (g) CI (g) Ionization Energies (kJ/mol) 418.8 1251.1 Electron Affinities (kJ/mol) -48.384 -349arrow_forward3. In general, as you go down a column in the periodic table, will the interaction between the nucleus and valence electrons get stronger or weaker? Explain. While you can simply memorize all the different periodic trends, many can be understood in terms of the two competing factors in Coulombs law ( Zeff vs. distance, r).arrow_forwardCombine two elements (cesium, berryllium, bromine, and flourine) from the following: ( K, Mg, Mn, N, O, Fe, Cu, H, S, Kr,C, Na, Br, Cl) to form 5 different molecules, including one oxy atomic moleculeand one diatomic molecule from the given elements(cesium, berryllium, bromine, and flourine), and determine the following: a) the electronegativity of the compound (show your calculation) b) Is the selected molecule polar, or not? What type of bond is formed based onwhat you learned? Is it a strong or weak bond?arrow_forward
- based on the following information calculate the approximate lattice energy of XCl2.X is 3x larger than Na+. The lattice energy for NaCl is 787kj/molearrow_forwardThe electron configuration of an element, X, is [Ne] 3s¹. The formula of the most probable ionic compound that this element will form with Br is Note: The compound Fe(CIO4)3 should be entered as "Fe(CIO4)3".arrow_forward1. Below is a list of enthalpy changes for the Born-Haber cycle for the formation of solid LiF from Li(s) and F(g). Use these data to determine the lattice energy for the formation LiF(s). Li(s) → Li(g) ΔH1 = +162 kJ/mol Li(g) → Li+(g) + e- ΔH2 = +520.2 kJ/molF2(g) → 2F(g) ΔH3 = 154 kJ/mol F(g) + e- → F-(g) ΔH4 = -328 kJ/molLi(s) + 1/2F2(g) → LiF(s) ΔHf = -612 kJ/mol Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a. 1371 kJ/mol b. -1371 kJ/mol c. 1043 kJ/mol d. -1043 kJ/molarrow_forward
- d. The energy required to separate the ions in the calcium carbonate crystal lattice into individual calcium and carbonate ions as represented in the table below is known as the lattice energy. As shown in the table, the lattice energy of magnesium carbonate is more than the lattice energy of calcium carbonate. Explain why in terms of periodic properties and Coulomb's law.arrow_forwardThe lattice energy of magnesium sulfide is the energy change accompanying the process Mg2*(g) + + S2-(g) → MgS(s) Calculate the lattice energy of MgS using the following data: Mg(s) → Mg(g) AH° = 148 kJ/mol Mg(g) → Mg2*(g) + 2e- AH° = 2186 kJ/mol Sg(s) → 8S(g) AH° = 2232 kJ/mol S(g) + 2e-- s2-(g) AH° = 450 kJ/mol 8Mg(s) + Sg(s) → 8MGS(s) AH° = -2744 kJ/mol Mg2*(g) + S2-(g)→ MgS(s) AH°lattice = ?arrow_forwardPlace the following elements in... increasing electronegativity: Se Cl S increasing ionization energy: Al P Sr increasing affinity for electrons: K Ge Cl increasing lattice energy: Na2S KCl MgSarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY