
Concept explainers
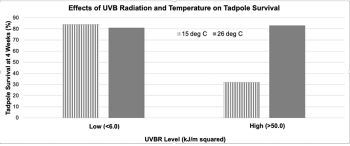
To conduct their experiment, the researchers collected L. peronii eggs from a natural stream. After the eggs had hatched, tadpoles were raised in artificially constructed 2 L containers in a laboratory. All containers were filled with 1 L of filtered tap water, and the water was changed every other day. Thirteen tadpoles were placed in each container, and the tadpoles were exposed to a 12-hour light: 12-hour dark photoperiod. All tadpoles were fed thawed spinach daily.
The researchers randomly placed the tadpoles into containers exposed to one of four treatments, with nine replicates of each treatment, as follows:
- Low UVBR (<6.0 kJ/m2), low temperature (15OC)
- Low UVBR (<6.0 kJ/m2), high temperature (26OC)
- High UVBR (>70.0 kJ/m2), low temperature (15OC)
- High UVBR (>70.0 kJ/m2), high temperature (26OC)
The researchers recorded the number of tadpoles still alive out of the original 13 in a container (percent survival) at different time periods. Their results at Week 4, showing the averages for each treatment, are shown below.
Figure 1. Graph of results for effects of temperature and UVB radiation on tadpole survival
Questions
- Identify all independent variables in the experiment (include units).
- Identify all dependent variables in the experiment (include units).
- Describe three standardized conditions in the experiment.
- Interpret the data in the graph shown in Figure 1. Be sure to address all of the treatments, if a treatment had an obvious effect or not, and what kind of effect it was.
Step by stepSolved in 2 steps

- The following data are for an F2 dihybrid corn ear. Calculate the x2 value (2 decimal points). Colored: C- Noncolored: cc Starchy: Su- Sweet: susu Data Phenotype O C-Su- 348 C-susu 119 ccSu- 139 ccsusu 42 Total Degrees of Freedom: _______ Accept or reject the null hypothesis: _______arrow_forwardfor clarification which is shown in the graph, the darker blue on the bar graph shows the mass(size) of the eggs and the striped blue represents the number of eggs which is also depicted in the graph. as far as the host plants, its just showing 3 different plants the butterflies were on which is represented by the a, b, and c, also located on the graph. The right side of the graph (mass) represents blue and the left side (number of eggs) represents the striped blue. part a: Describe the relationship between egg size and clutch size shown in Figure 1. (This is not asking about host plants, just about the butterfly eggs.) part b: Describe one possible reason for the relationship between egg size and clutch size observed. (This is not asking about the host plants, just about the butterfly eggs.) part c:Describe one realistic difference between host plants that might be affecting the numbers and sizes of eggs that a female lays on these plants.arrow_forwardWhat type of mating system developed in Round 1 without male parental involvement? Why? What type of mating system developed in Round 2 with male parental involvement? Why? If males and females had the same number of sperm, would the two rounds differ? howarrow_forward
- Convert the following data table into a venn diagram and then into a cladogram (refer to the image):arrow_forwardPlease solve step by step THANKS! A researcher when studying insects of the genus Protenor, crossed females with normal wings and short antennae, with males with short wings and long antennae. In the first generation, he found that all insects had normal wings and long antennae; Therefore, when obtaining the second generation of a count of 460 insects, 50 presented short wings and short antennae; 135 with normal wings and short antennae; 165 with short wings and long antennae; and 465 with normal wings and long antennae. According to the information, determine:a) The genotype of the F1 and the parentsb) How many pairs of genes are involved in the crossc) How is the inheritance of these characters,d) Obtain the genotypic and phenotypic relationshipe) If the test cross is made, what genotypic and phenotypic relationship is expectedarrow_forwardTo directly test if multicellularity in yeast can evolve as a response to predation pressure, what kind of experiment should you conduct? Question 2 options: Cultivate unicellular yeast in the presence and absence of predators and compare the frequency multicellular clusters in each treatment Add rotifers to pure cultures of unicellular and multicellular yeast and measure the change in light absorption between unicellular and multicellular cultures over 5 days Cultivate unicellular and multicellular yeast in the presence of predators and compare their growth rate Cultivate unicellular and multicellular yeast under the exact same conditions and compare their fitnessarrow_forward
- What is the Red Queen Dynamic? Using the snail example, explain why different parasite and host genotypes are expected to oscillate in frequency? Why would this favor sexual reproduction or outcrossing?arrow_forwardDescribe the concept of the Red Queen Hypothesis in your own words.arrow_forwardWhat is one challenge posed by copulation between separate sexes? What are some adaptations that grasshoppers have developed that address these challenges?arrow_forward
- You are examining a population of C. elegans in their natural habitat, a fresh steaming pile of nutrient-rich compost. Predict what you might expect to see in terms of mode of reproduction in the population. a. The population will primarily reproduce asexually then when they run out of nutrient-rich compost. b. The population will have mostly hermaphrodites with a few males. c. The population will have a really high frequency of males compared to lab conditions. d. The population will primarily undergo sexual reproduction.arrow_forwardGrasshopper's environment pressures: What obstacles in regard to finding food, habitat, climate, mating? How does it deal with these pressures?arrow_forwardHoney bee workers from a colony in North Carolina are visiting two food sites, A and B, at 6 am as shown below. Site A is 1500 m from the hive; site B is 300 m from the hive. Which of the following statements is true regarding the genetic basis of the bees’ ability to track the passage of time and compensate for the movement of the sun? a. The bees’ clock mechanism is based on the Amfor gene, which causes a build up of AMFOR protein in the corpora pedunculata that activates the Gp9 and UU genes, whose products degrade AMFOR in a 24 hour cycle. b. The bees’ clock mechanism is based on the per gene in the optic lobes, whose activity has a 24 hour cycle regulated by the dbt and tim genes. c. The bees’ clock mechanism is based on alternating expression of two alleles for the for gene in the subesophagael ganglion: the R allele which is active at night and the s allele which is active during the day. d. The bees’ clock mechanism is based on levels of eclosion hormone released from…arrow_forward
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





