
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:ts
eBook
Print
ferences
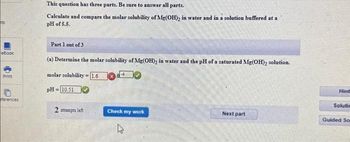
This question has three parts. Be sure to answer all parts.
Calculate and compare the molar solubility of Mg(OH)₂ in water and in a solution buffered at a
pH of 5.5.
Part 1 out of 3
(a) Determine the molar solubility of Mg(OH)₂ in water and the pH of a saturated Mg(OH)₂ solution.
molar solubility
1.6
pH=10.51
2 attempts left
B
Check my work
Next part
Hint
Solutia
Guided So
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- calculate the molar solubility of AgBr in 0.10 M NaBr solution.arrow_forwardFor this question Find the initial pH of 0.2000 L of buffer solution that is 0.165 M acetic acid and 0.145 M sodium acetate, and calculate the pH after the addition of 16.00 mL of 0.650 M potassium hydroxide.arrow_forward[References] Use the References to access important values if needed for th Which of the following aqueous solutions are good buffer systems? 0.36 M barium iodide + 0.29 M calcium id lide 0.19 M hydrocyanic acid + 0.19 M potassium cyanide 0.20 M sodium hydroxide + 0.27 M sodium bromide 0.29 M ammonia + 0.36 M barium hydroxide 0.27 M perchloric acid + 0.23 M potassium perchlorate Submit Answer Try Another Version 1 item attempt remainingarrow_forward
- Use the References to access important values if needed for this question. Which of the following aqueous solutions are good buffer systems? 0.28 M ammonia + 0.35 M calcium hydroxide . 0.11 M potassium hydroxide + 0.23 M potassium bromide . 0.39 M sodium perchlorate + 0.23 M potassium perchlorate . 0.15 M hypochlorous acid + 0.13 M potassium hypochlorite . 0.27 M hydroiodic acid + 0.25 M potassium iodide .arrow_forwardA chemistry graduate student is given 450. mL of a 1.70M hydrocyanic acid (HCN) solution. Hydrocyanic acid is a weak acid with K,= 4.9 × 10 ". What mass of KCN should the student dissolve in the HCN solution to turn it into a buffer with pH = 8.97? You may assume that the volume of the solution doesn't change when the KCN is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits.arrow_forwardA chemistry graduate student is given 300. mL of a 0.80 M methylamine (CH,NH,) solution. Methylamine is a weak base with K, = 4.4 x 10 . what mass of CH,NH,Cl should the student dissolve in the CH,NH, solution to turn it into a buffer with pH = 10.92? You may assume that the volume of the solution doesn't change when the CH.NH,Cl is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. Continuearrow_forward
- Please don't image upload answer type in word thank you.arrow_forwardA chemistry graduate student is given 250. mL of a 1.40M trimethylamine ((CH,) N) solution. Trimethylamine is a weak base with K, =7.4 × 10 *. What 3 mass of (CH,) NHCI should the student dissolve in the (CH, N solution to turn it into a buffer with pH =10.85? 3 You may assume that the volume of the solution doesn't change when the (CH,) NHC1 is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. olo x10 Ar ? Explanation Check © 2022 McGraw Hill LLC. AlL Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardIf 100.0 g calcium phosphate (Ksp= 1.3 x 10^-32) are placed in enough water to generate 750.0 mL of solution, calculate the molar solubility of the calcium phosphate solution and the molar solubility of the calcium ion if 30.0 mL of 0.400 M calcium nitrate solution is added to the original solution.arrow_forward
- 3. Please solve the following chemistry problem. arrow_forwardA chemistry graduate student is given 250. mL of a 1.80 M dimethylamine ((CH3), NH) solution. Dimethylamine is a 2 -4 weak base with K = 5.4 × 10 What mass of (CH, NH,Br should the student dissolve in the (CH,) NH solution to 2 turn it into a buffer with pH = 11.26? You may assume that the volume of the solution doesn't change when the (CH,) NH,Br is dissolved in it. Be sure your 2 answer has a unit symbol, and round it to 2 significant digits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY