
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
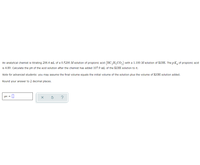
Transcribed Image Text:An analytical chemist is titrating 206.4 mL of a 0.5200 M solution of propionic acid (HC,H,CO,) with a 1.100 M solution of KOH. The p K, of propionic acid
is 4.89. Calculate the pH of the acid solution after the chemist has added 107.9 mL of the KOH solution to it.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of KOH solution added.
Round your answer to 2 decimal places.
pH =
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An analytical chemist is titrating 68.1 mL of a 0.6500M solution of propionic acid (HC,H,CO,) with a 0.4500M solution of KOH. The p K, of propionic acid is 4.89. Calculate the pH of the acid solution after the chemist has added 114. mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of KOH solution added. Round your answer to 2 decimal places.arrow_forwardAn analytical chemist is titrating 108.9 mL of a 0.8900 M solution of propionic acid (HC,H,CO,) with a 1.000 M solution of NaOH, The p K of propionic acid is 4.89. Calculate the pH of the acid solution after the chemist has added 73.48 mL of the NaOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of NaOH solution added. Round your answer to 2 decimal places. pH = Check Explanation rivacy Accessibility Terms 2021 McGravw.Hill Education. All Rights Reserved V I 10: cerarrow_forwardneed help with this chemistryarrow_forward
- An analytical chemist is titrating 185.8 mL of a 1.200M solution of benzoic acid (HC,H,CO,) with a 1.200M solution of KOH. The p K, of benzoic acid is 4.20. Calculate the pH of the acid solution after the chemist has added 196.6 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of KOH solution added. Round your answer to 2 decimal places. olo pH = ] Ar G Explanation Check © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility ............................... .................................. .............. .... .arrow_forwardA chemist titrates 100.0 mL of a 0.3992 M ethylamine (C,H;NH,) solution with 0.6385 M HCl solution at 25 °C. Calculate the pH at equivalence. The p K, of ethylamine is 3.19. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HCl solution added. pH =arrow_forwardA chemist titrates 150.0 mL of a 0.0676M dimethylamine ((CH,) NH) solution with 0.4402M HBr solution at 25 °C. Calculate the pH at equivalence. The pK, of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = Explanation Check 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Accessibity MacBook Airarrow_forward
- An analytical chemist is titrating 119.2 mL of a 0.1800M solution of ammonia (NH,) with a 0.4500M solution of HNO2. The p Kb of ammonia is 4.74. Calculate the pH of the base solution after the chemist has added 53.9 mL of the HNO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added. 3 Round your answer to 2 decimal places. pH ?arrow_forwardAn analytical chemist is titrating 74.4 mL of a 0.3100 M solution of butanoic acid (HC,H,CO,) with a 0.3400 M solution of KOH, The p K_ of butanoic acid is 4.82. Calculate the pH of the acid solution after the chemist has added 72.3 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of KOH solution added. Round your answer to 2 decimal places. do pH = 0 Pvacy Accessibilty Check 2021 McGraw-Hill Education All Rights Reserved Terms of Use Explanation V 11:16arrow_forwardA chemist titrates 90.0 mL of a 0.6900M ethylamine (C,H,NH,) solution with 0.0547M HNO, solution at 25 °C. Calculate the pH at equivalence. The p K of ethylamine is 3.19. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HNO, solution added. pH = da Explanation Check O 2021 McGraw-H Education All Rights Reserved Terms of Use Pvacy Accessibity 會 APR 23 MacBook Air 20 888 FS esc & delete $ 8 5 2 E R T Y Q W F Garrow_forward
- Calculating the pH of å Weak Base lilla An analytical chemist is titrating 61.7 mL of a 0.3400M solution of aniline (CH,NH, with a 0.8400M solution of HIO3. The p K, of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 10.9 mL of the HIO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HIO, solution added. Round your answer to 2 decimal places. dh pH = Explanation Check 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Accessibility 24 MacBook Airarrow_forwardAn analytical chemist is titrating 234.0 mL of a 0.4400M solution of butanoic acid (HC,H,CO,) with a 0.3900M solution of NaOH. The p K, of butanoic acid is 4.82. Calculate the pH of the acid solution after the chemist has added 63.61 mL of the NaOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of NaOH solution added. Round your answer to 2 decimal places.arrow_forwardA chemist titrates 110.0 mL of a 0.7452M aniline (C,H,NH,) solution with 0.5088M HCl solution at 25 °C. Calculate the pH at equivalence. The p K, of aniline is 4.87. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HCl solution added. pH = ||arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY