
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please don't provide handwritten solution ...
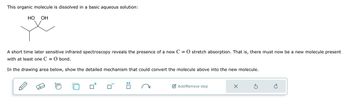
Transcribed Image Text:This organic molecule is dissolved in a basic aqueous solution:
HO
OH
A short time later sensitive infrared spectroscopy reveals the presence of a new C = O stretch absorption. That is, there must now be a new molecule present
with at least one C = O bond.
In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule.
Add/Remove step
☑
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- This organic molecule is dissolved in a basic aqueous solution: A short time later sensitive infrared spectroscopy reveals the presence of a new C-OH stretch absorption. That is, there must now be a new molecule pre with at least one C-OH bond. In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule.arrow_forwardUse the Text Submission box to answer the following question. Consider the reaction shown below. For a particular concentration of sodium methoxide, the absorbance at 244 nm is measured using UV-vis spectroscopy. Over an initial period, the absorbance at 244 nm increases from 0.50 to 0.60. When the concentration of sodium methoxide is doubled, the absorbance at 244 nm measured after the same initial period is 0.70. What can you conclude about the mechanism of the reaction? NaOCH 3 Amax = 244 nmarrow_forwardshow the mechanism step for this (i dont want hand writting image)arrow_forward
- Give detailed mechanism Solution with explanation needed. Don't give Handwritten answerarrow_forwardplease give a mechanism C C ง NH2arrow_forwardAfter running various experiments, you determine that the mechanism for the following reaction occurs in a step-wise fashion. Br + + ག ཡིག་ ཐ དང༠ པ—- ིད་ཡིག་ - དང་ ལུ ད + Using this information, draw the correct mechanism in the space below. Br Br * : ☐ ? g Add/Remove step ☑ 000 Click and drag to start drawing a structure. 18 Ar B كاarrow_forward
- ( plz do all plz and will upvote with explanation and mechanism plz give mechanismarrow_forwardThis organic molecule is dissolved in an acidic aqueous solution: i A short time later sensitive infrared spectroscopy reveals the presence of a new C. with at least one C - OH bond. - OH stretch absorption. That is, there must now be a new molecule present In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule. : Click and drag to start drawing a structure. Add/Remove step Click and drag to start drawing a structure.arrow_forwardShown below is a carbocation intermediate in an electrophilic addition reaction of HCl with an alkene. For the conformation shown, select each hydrogen whose bond to carbon is aligned for effective hyperconjugation with the vacant p orbital on the positively charged carbon. Each adjacent carbon will have only one C-H bond so aligned. • Gray = C; white = H; red = 0; blue = N; dark green = Cl; brown =Br; light green =F; purple = I; yellow = S; orange = P. • Double click to select atoms. • You can zoom in and out using the mouse scroll wheel (or pinch to zoom on touch screens).arrow_forward
- Show the electron-flow mechanism of the following. This involves predicting major and by-products using electronic and structural effect CH3 CI. `AI C=c-H H-CI 1 %3D + + + H H. CI Br CH3 + N-Br + Br-Br = CH3 12 Br CH3 CH2 + HBr %3D 3. || I Iarrow_forwardGive detailed mechanism Solution with explanation needed. Don't give Handwritten answer..don't copy the answer anywherearrow_forwardPlease provide a mechanismarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
