
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Use the graph to answer the following questions:
Which liquid is the most volatile?
Which is the least volatile?
What is the normal boiling point of each liquid?
Note: your answer must be within 1°C of the exact
answer
Suppose a beaker of pyrrole is put inside a sealed
tank containing pyrrole gas at 122. degree C and
470. torr. After ten minutes, will there be more liquid
in the beaker, less liquid, or the same amount?
most volatile:
least volatile:
piperidine:
pyrrole:
orthoxylene:
more
less
the same
choose one î
choose one
°℃
°℃
°℃
X
î
Ś
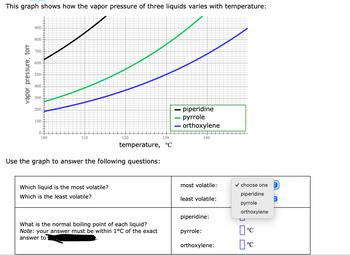
Transcribed Image Text:This graph shows how the vapor pressure of three liquids varies with temperature:
vapor pressure, torr
900-
800-
700
600-
500
400.
300
200.
100.
0
100
110
120
130
temperature, °C
Use the graph to answer the following questions:
Which liquid is the most volatile?
Which is the least volatile?
What is the normal boiling point of each liquid?
Note: your answer must be within 1°C of the exact
answer to
- piperidine
pyrrole
orthoxylene
140
most volatile:
least volatile:
piperidine:
pyrrole:
orthoxylene:
✓ choose one
piperidine
pyrrole
orthoxylene
0°
Пос
°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the normal boiling point of each compound? Vapor pressure (mm Hg) 800 700 600 500 400 300 200 100 0 -30 -10 CS₂: CH3 NO2: °℃ °C 10 CS₂ 50 30 Temperature (°C) CH3NO₂ 70 90 110arrow_forwardUse the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 3.7 atm. pressure (atm) 0- 8 solid liquid gas 100 temperature (K) 200 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. ☐ °C Garrow_forward1arrow_forward
- This question as been getting rejected, but this is not a grade assigment. This just a pratice problem.arrow_forwardThis graph shows how the vapor pressure of three liquids varies with temperature: vapor pressure, torr 900- 800- 700- 600 500 400. 300 200 100. 0+ 100 130 temperature, °C Use the graph to answer the following questions: 110 120 Which liquid is the most volatile? Which is the least volatile? What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be Suppose a beaker of isobutyl alcohol is put inside a sealed tank containing isobutyl alcohol gas at 106. degree C and 701. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? ▪ethylbenzene - isobutyl alcohol orthoxylene 140 most volatile: least volatile: ethylbenzene: isobutyl alcohol: orthoxylene: more less the same ✓ choose one ethylbenzene isobutyl alcohol orthoxylene °C °Carrow_forwardThe vapor pressure of Substance X is measured at several temperatures: temperature vapor pressure 4. °C 0 16. °C 28. °C 0.0584 atm 0.0961 atm 0.152 atm Use this information to calculate the enthalpy of vaporization of X. Round your answer to 2 significant digits. Be sure your answer contains a correct unit symbol. x10 ロ・ロ X I olo Śarrow_forward
- Which of these would have the lowest boiling pointarrow_forwardPlease answer all parts 1,2 and 3arrow_forwardConsider the following phase diagram. Phase diagram for compound A 1000 2 760 3 50 10 -25 25 100 150 Temperature, °C (not to scale) Which red dot represents the melting point of this compound at 760 torr? [ Select ] What is the approximate melting temperature for this substance at 760 torr? [ Select ] Which red dot represents the boiling point of this compound at 760 torr? [ Select ] What is the approximate boiling temperature for this substance at 760 torr? [ Select ] Which red dot represents the triple point for this substance? [ Select ] |What is the approximate temperature of the triple point? [ Select ] Pressure, torr (not to scale)arrow_forward
- This graph shows how the vapor pressure of three liquids varies with temperature: 900. 800 700 600 500 400 300 200. ethylbenzene octane 100 acetic acid 0キ 100 110 120 130 140 temperature, °C Use the graph to answer the following questions: most volatile: choose one Which liquid is the most volatile? Which is the least volatile? least volatile: choose one °C ethylbenzene: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. °C octane: acetic acid: °C more Suppose a beaker of octane is put inside a sealed tank containing octane gas at 123. degree C and 818. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? less O the same vapor pressure, torrarrow_forwardThis graph shows how the vapor pressure of three liquids varies with temperature: vapor pressure, torr 900- 800 700 600- 500 400- 3001 200 1002 0. 100 110 120 Which liquid is the most volatile? Which is the least volatile? 130 temperature, °C Use the graph to answer the following questions: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be -ethylbenzene -acetic acid pyrrole Suppose a beaker of acetic acid is put inside a sealed tank containing acetic acid gas at 104. degree C and 478. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? 140 most volatile: least volatile: ethylbenzene: acetic acid: pyrrole: O more O less O the same choose one choose one °C 0 °℃ °℃ X ✪ ✪arrow_forwardPlease don't provide handwritten solutionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY