
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
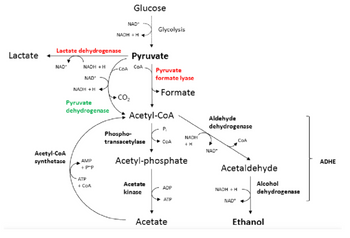
Transcribed Image Text:Lactate +
Lactate dehydrogenase
NAD+
NADH + H
NAD+
NADH + H
Pyruvate
dehydrogenase
Acetyl-CoA
synthetase
AMP
+ P*P
ATP
+ CoA
NADH + H
-COA
Glucose
NAD+
CO₂
Pyruvate
COA
Glycolysis
Phospho-
transacetylase
Acetyl-CoA
Pyruvate
formate lyase
Formate
Acetate
kinase
P₁
COA
Acetyl-phosphate
ADP
ATP
NADH
+ H
Acetate
Aldehyde
dehydrogenase
NAD*
Acetaldehyde
NADH + H
COA
NAD+
Alcohol
dehydrogenase
Ethanol
ADHE
Transcribed Image Text:10_UG_26563_ch09_167-184.indd 175
Press esc to exit full screen
Yeast Fermentation
The unicellular fungus Saccharomyces cerevisiae is used in the food industry to ferment sugars.
The rate of fermentation is measured by CO₂ production. The addition of inorganic phosphate
increases this rate.
THINK Answer this question individually:
1. Why does the fermentation of sugars, such as glucose and fructose, depend upon the
presence of P?
PAIR Answer the following questions with a partner:
2. Which glycolytic reactions require P?
3. Several glycolytic intermediates contain phosphate. Where did these groups come from
during the reactions?
SHARE
4. How much P, is required to generate one CO₂?
As a class, discuss the following questions:
5. Is CO₂ generation the most reliable way to measure ethanol fermentation?
Why or why not?
HANDOUT
175
23/03/17 2:02 pm
Page
1
of 4
......
ZOOM +
K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Plz explain and give the correct answerarrow_forwardGive typed explanation of both otherwise leave itarrow_forwardin am since cOz is already 7) a) What are the molecular products of glycolysis, and what is the net amount of them that you would obtain from glycolysis per glucose? RULED PAGES LICarrow_forward
- Please answer correctlyarrow_forwardConsider the following redox reaction: lactate + NAD = pyruvate + NADH + H+ The relevant reductive half reactions are as follows: pyruvate + 2e + 2H+ lactate NAD+ + 2e+ H* = NADH E' -0.185 V = E' -0.320 V What is the AE' of the redox reaction? -0.505 V -0.135 V 0.135 V ○ 0.505 Varrow_forward2.1 ANSWER THE TABLEarrow_forward
- In the skeletal muscle, in anaerobic conditions, glyceraldehyde 3-phosphate is converted into pyruvate during the payoff phase of glycolysis; and this pyruvate is reduced into lactate during lactic fermentation. Part 1-Write the 11 balanced biochemical equations corresponding to all the reaction steps leading to the conversion of glyceraldehyde-3-phosphate into lactate through glycolysis followed by lactic fermentation. Part 2-Write the net equation of the whole transformation process (i.e. with glyceraldehyde-3-phosphate as the starting substrate; and lactate as the end product).arrow_forwardPropose structures for intermediates A and B in the scheme below. This three-step conversion is carried out by enzymes that require no redox cofactors (no FAD, NADH etc.) nor TPP. Which enzyme in the citric acid cycle has an activity most similar to enzyme 1? Which enzyme in the PPP or the citric acid cycle has an intermediate that bears similarity to intermediate B? H OH COO™ Enzl A Enz2 B Enz3 OHarrow_forwardWhich of the following about glycolysis are CORRECT? 1 I. II. III. IV. Among all the reactions in glycolysis, reaction catalysed by hexokinase has the largest negative AG The overall pathway of glycolysis has a negative AG Glycolysis is tightly regulated in coordination with other energy-yielding pathways Two NADP molecules are released and coupled exergonic reactions possible. A. I and II B. I and III O C. II and IV D. I, II and III E. All of themarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON