
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
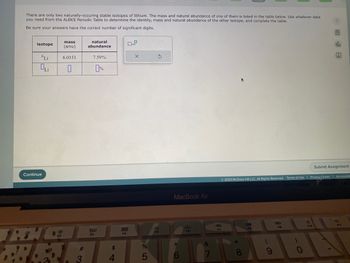
Transcribed Image Text:There are only two naturally-occuring stable isotopes of lithium. The mass and natural abundance of one of them is listed in the table below. Use whatever data
you need from the ALEKS Periodic Table to determine the identity, mass and natural abundance of the other isotope, and complete the table.
Be sure your answers have the correct number of significant digits.
isotope
'Li
Oli
Continue
F2
mass
(amu)
6.0151
natural
abundance
7.59%
0%
80
F3
888
F4
$
PRAAK
%
5
S
F5
MacBook Air
A
F6
&
7
B
F7
*
8
DII
FO
Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibili
)
?
F10
F
ol
Ar
Submit Assignment
F
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An unknown element is determined to have two naturally occurring isotopes. Isotope 1 is 68.11% abundant and has a mass of 68.925580 amu, and isotope 2 has a mass of 70.9247005 amu. Determine the atomic mass of the unknown element. Express your answer to four significant figures.arrow_forwardThere are only two naturally-occuring stable isotopes of chlorine, the masses of which are listed in the table below. Use whatever data you need from the ALEKS Periodic Table to calculate the natural abundance of each isotope and complete the table. 35 Round your entry for C1 to 4 significant digits and your entry for isotope 35 Cl 37 Cl mass (amu) 34.969 36.966 natural abundance 1% % ☐x10 X 37 C1 to 4 significant digits. Śarrow_forwardAn element has two naturally occurring isotopes. One has an abundance of 51.839% and an isotopic mass of 106.905093 AMU. The other has an abundance of 48.161% and an isotopic mass of 108.904756 AMU. What is the average atomic mass of the element?arrow_forward
- There are only two naturally-occuring stable isotopes of antimony. The mass and natural abundance of one of them is listed in the table below. Use whatever data you need from the ALEKS Periodic Table to determine the identity, mass and natural abundance of the other isotope, and complete the table. Be sure your answers have the correct number of significant digits. isotope Osb 123 Sb mass (amu) 122.90 natural abundance % 42.79% x10 X Śarrow_forwardThere are only two naturally-occuring stable isotopes of lithium. The mass and natural abundance of one of them is listed in the table below. Use whatever data you need from the ALEKS Periodic Table to determine the identity, mass and natural abundance of the other isotope, and complete the table. Be sure your answers have the correct number of significant digits. isotope Qui Li mass (amu) 0 7.0160 natural abundance [% 92.41% x10 X Śarrow_forwardAn atom has a diameter of 2.50 Å and the nucleus of that atom has a diameter of 6.00 x 10-5 Å. Determine the fraction of the volume of the atom that is taken up by the nucleus. Assume the atom and the nucleus are a sphere. fraction of atomic volume: Calculate the density of a proton, given that the mass of a proton is 1.0073 amu and the diameter of a proton is 1.69 × 10-¹5 density: m. g/cm³arrow_forward
- A robot spacecraft returned samples from the planetesimal 98765 ALEKS, located in the outer Solar System. Mass-spectroscopic analysis produced the following data on the isotopes of tin in these samples: mass relative isotope (amu) abundance 112 Sn 90.3% 111.9 119 Sn 9.7% 118.9 Use these measurements to complete the entry for tin in the Periodic Table that would be used on 98765 ALEKS. Round your entry for the atomic mass to 3 significant digits. Caution: your correct answer will have the same format but not necessarily the same numbers as the entry for tin in the Periodic Table we use here on Earth. Snarrow_forwardA new element has been discovered with two isotopes having masses of 84.63 amu and 90.63 amu. The average mass is 88.01 amu. Write the isotopic atomic mass that is the most abundant.arrow_forwardA robot spacecraft returned samples from the planetesimal 98765 ALEKS, located in the outer Solar System. Mass-spectroscopic analysis produced the following data on the isotopes of molybdenum in these samples: mass relative isotope (amu) abundance 96- Мо 95.9 41.% 91 Mo 59.% 96.9 Use these measurements to complete the entry for molybdenum in the Periodic Table that would be used on 98765 ALEKS. Be sure your answers have the correct number of significant digits. Caution: your correct answer will have the same format but not necessarily the same numbers as the entry for molybdenum in the Periodic Table we use here on Earth. Moarrow_forward
- 1- For each of the following atoms, calculate the number of protons and neutrons in the nucleus and the number of electrons outside the nucleus (assume neutral atoms). element number of number of number of electrons mass number (A) symbol 126C protons (Z) neutrons 12 10 12 10 14 27 10 23,1Na 11 17 18 2- Calculate the atomic mass to four significant figures for carbon, given the following data: Isotope Exact Atomic Mass (amu) 12.00000 13.00335 Abundance in Nature (%) 98.89 1.110 12C 13Carrow_forwardA robot spacecraft returned samples from the planetesimal 98765 ALEKS, located in the outer Solar System. Mass-spectroscopic analysis produced the following data on the Isotopes of ruthenium in these samples: mass relative isotope (amu) abundance 100 Ru 99.90 58.% 96 Ru 95.91 42.% Use these measurements to complete the entry for ruthenium in the Periodic Table that would be used on 98765 ALEKS. Round your entry for the atomic mass to 2 significant digits. Caution: your correct answer will have the same format but not necessarily the same numbers as the entry for ruthenium in the Periodic Table we use here on Earth. Ru Submit Assignment Continue 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility DII -> esc & 23 $4 @ 4 3 rarrow_forwardThe atomic number indicates_ the total number of neutrons and protons in a nucleus the number of different isotopes of an element the number of neutrons in a nucleus O the number of atoms in 1 g of an element the number of protons or electrons in a neutral atomarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY