
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
![**Vapor Pressure in Binary Solutions**
The vapor pressures of the components, \( A \) and \( B \), in a binary solution have been modeled and found to obey the following equations:
\[
P_A = \chi_A P_A^* \exp(\chi_B^2)
\]
\[
P_B = \chi_B P_B^* \exp(\chi_A^2)
\]
where \( \chi_A \) and \( \chi_B \) are the mole fractions, and \( P_A^* \) and \( P_B^* \) are the vapor pressures of each pure substance.
### Problems
**(a)** If \( P_A^* = 0.175 \, \text{bar} \) and \( P_B^* = 0.126 \, \text{bar} \), compute the total vapor pressure (in bars) of the mixture when \( \chi_B = 0.68 \).
**(b)** Assuming that the vapor is an ideal gas, what are the mole fractions of each component in the vapor phase?](https://content.bartleby.com/qna-images/question/3c924432-5356-4d2a-9799-f03f3a53c4ab/aa992869-7bc0-4acd-a4fa-77abaf08dc56/kkk4fjq_thumbnail.png)
Transcribed Image Text:**Vapor Pressure in Binary Solutions**
The vapor pressures of the components, \( A \) and \( B \), in a binary solution have been modeled and found to obey the following equations:
\[
P_A = \chi_A P_A^* \exp(\chi_B^2)
\]
\[
P_B = \chi_B P_B^* \exp(\chi_A^2)
\]
where \( \chi_A \) and \( \chi_B \) are the mole fractions, and \( P_A^* \) and \( P_B^* \) are the vapor pressures of each pure substance.
### Problems
**(a)** If \( P_A^* = 0.175 \, \text{bar} \) and \( P_B^* = 0.126 \, \text{bar} \), compute the total vapor pressure (in bars) of the mixture when \( \chi_B = 0.68 \).
**(b)** Assuming that the vapor is an ideal gas, what are the mole fractions of each component in the vapor phase?
Expert Solution
arrow_forward
Step 1 Formula
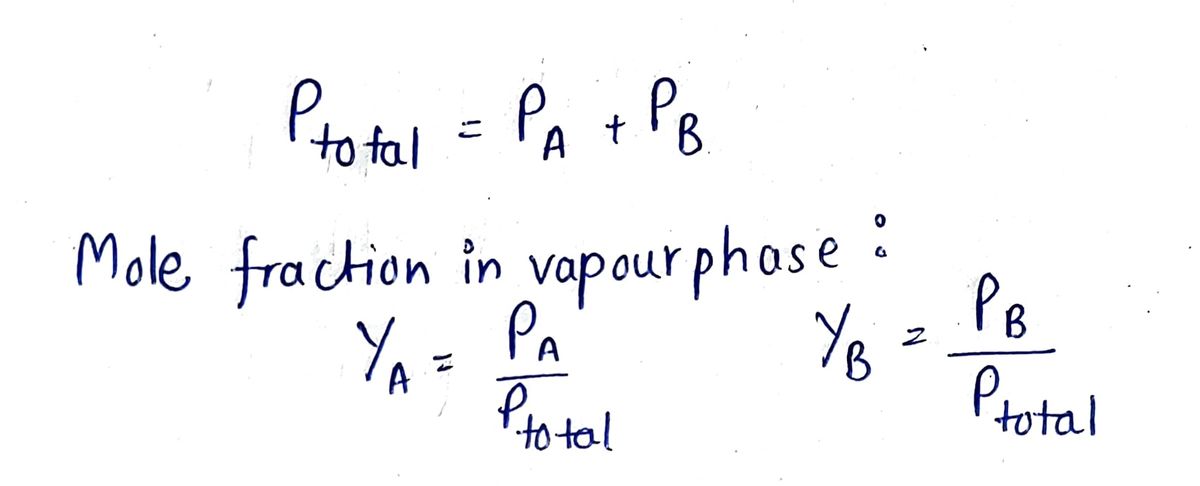
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you please answer number 8 and show all of the steps to the solutionarrow_forwardAssuming 100% dissociation, calculate the freezing point (T;) and boiling point (T) of 2.32 m Na,SO,(aq). Colligative constants can be found in the chempendix. T; °C Ть °C || ||arrow_forward2) Draw the isobaric phase diagram at 1 atm of the following two-component liquid system: water (Boiling point = 100°C) and nitric acid (Boiling point = 86°C), with the mass percentage of nitric acid on the x-axis. When the liquid mixture contains 68 % nitric acid, an azeotrope with a boiling point of 120.5°C, is formed. Use the sketch to describe under which conditions pure nitric acid can be obtained by means of isobaric distillation.arrow_forward
- The graph given below plots the (approximate) partial molar volumes of ethanol (black line) and water (red line) in units of ml/mole at 25°C in a mixture as a function of the mole fraction of ethanol. Use the information in this graph (note the different y axes for water and ethanol) and the densities at 25°C to calculate the resulting total volume of the g following two mixtures.(pethanol = 0.789 ml & PH20 = 0.998 ) ml a. 150 ml ethanol + 100 ml H2O b. 200 ml ethanol + 40 ml H2O H20 VELOH Vet o*HA -V ELOH V H2O T 0.8 1.0 0.2 0.4 0.6 0.0 XELOH 53 54 55 57 15 18arrow_forwardwe are calculating the molar mass from the freezing point depression. What would the effect be on calculated molar mass if the thermometer was not properly calibrated andconsistently reads 1.3oC too high?arrow_forwardGive typed solutionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY