
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
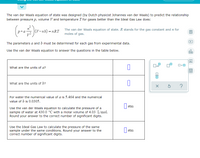
Transcribed Image Text:The van der Waals equation of state was designed (by Dutch physicist Johannes van der Waals) to predict the relationship
between pressure p, volume V and temperature T for gases better than the Ideal Gas Law does:
p+a-
|(V-nb) = nRT The van der Waals equation of state. R stands for the gas constant and n for
moles of gas.
The parameters a and b must be determined for each gas from experimental data.
Use the van der Waals equation to answer the questions in the table below.
Ar
What are the units of a?
What are the units of b?
For water the numerical value of a is 5.464 and the numerical
value of b is 0.0305.
Oat
||atm
Use the van der Waals equation to calculate the pressure of a
sample of water at 430.0 °C with a molar volume of 4.03 L/mol.
Round your answer to the correct number of significant digits.
Use the Ideal Gas Law to calculate the pressure of the same
sample under the same conditions. Round your answer to the
correct number of significant digits.
atm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- According to the ideal gas law, a 9.556 mol sample of argon gas in a 0.8165 L container at 503.4 K should exert a pressure of 483.5 atm. What is the percent difference between the pressure calculated using the van der Waals' equation and the ideal pressure? For Ar gas, a = 1.345 L2atm/mol2 and b = 3.219×10-2 L/mol. what is the percent difference?arrow_forwardHeptane (C7H16) gas reacts with oxygen gas to give carbon dioxide gas and water vapor. If you mix heptane and oxygen in the correct stoichiometric ratio to make the reaction go to completion without any excess reagent, and if the total pressure of the mixture is 705 mmHg, what is the partial pressure of heptane? Enter your answer in units of mmHg.arrow_forwardThe van der Waals equation of state was designed (by Dutch physicist Johannes van der Waals) to predict the relationship between pressure p, volume Vand temperature T for gases better than the Ideal Gas Law does: p+a (V-nb)=nRT The van der Waals equation of state. R stands for the gas constant and n for moles of gas. The parameters a and b must be determined for each gas from experimental data. Use the van der Waals equation to answer the questions in the table below. What are the units of a? What are the units of b? For argon the numerical value of a is 1.337 and the numerical value of b is 0.0320. D atm Use the van der Waals equation to calculate the pressure of a camnle of argon at –1100 °C with a molar volume of 口口arrow_forward
- A 7.13 L cylinder contains 1.82 mol of gas A and 3.77 mol of gas B, at a temperature of 31.8 °C. Calculate the partial pressure of each gas in the cylinder. Assume ideal gas behavior. PA= PB = What is the total pressure? PTotal = kPa kPa kPaarrow_forwardA 44.0 L metal cylinder stores a sample of gas at 6 °C under 1.02 atm. The temperature of the storage unit rises to 76 °C, causing the gas to decompose and doubling the number of moles in the cylinder. Note: R = 0.08206 Latm/molK Determine the moles of gas in the cylinder before any decomposition occurs. Determine the pressure in the cylinder after the decomposition in atmospheres.arrow_forwardStarting with Charles's law (stated as an equation), obtain an equation for the final volume of a gas from its initial volume when the temperature is changed at constant pressure. From Charles's law, VT = constant Because this is true for initial conditions as well as for final conditions, we can write V. V; T T; Thus, the equation for the final volume is P.V = P;V; Drag and drop your selection from the following list to complete the answer: P T; V, = V, x Pr PV = constant V = constamt T VT = V;T, V, = V, x T = constant V. P;V; P V, = V, x P, PV = constant T Tarrow_forward
- Consider the reaction, 2 Al (s) + 6 HCI (aq) → 2 AICI, (aq) + 3 H, (g). When 40.0 g of Al react with an excess of HCl at 25.0 °C the pressure in a collection flask is measured to be 1.50 atm. What is the volume of the collection flask?arrow_forwardAccording to the ideal gas law, a 1.042 mol sample of methane gas in a 1.670 L container at 267.2 K should exert a pressure of 13.68 atm. What is the percent difference between the pressure calculated using the van der Waals' equation and the ideal pressure? For CH, gas, a = 2.253 L²atm/mol2 and b = 4.278×10-2 L/mol. Pideal - Pvan der Waals| Percent difference = × 100 Pideal +Pvan der Waals 2)arrow_forwardSuppose 10.05 mol of Ar gas are pumped into a 1.56 L container at 293.15 K. Calculate the expected pressure based on the ideal gas law, Pideal. Calculate an estimate of the gas pressure one might observe based on the van der Waals equation, Pobs.a = 1.34 L2 · atm/mol2, b = 0.0322 L/mol.arrow_forward
- According to the ideal gas law, a 10.24 mol sample of xenon gas in a 0.8381 L container at 499.4 K should exert a pressure of 500.7 atm. What is the percent difference between the pressure calculated using the van der Waals' equation and the ideal pressure? For Xe gas, a 4.194 L²atm/mol² and b = 5.105×102 L/mol. = Percent difference | Pideal – Pvan der Waals | × 100 Pideal+Pvan der Waals % 2arrow_forwardA 10.00 L tank at 5.3 °C is filled with 5.84 g of carbon dioxide gas and 4.39 g of chlorine pentafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction of each gas. Be sure each of your answer entries has the correct number of significant digits. gas carbon dioxide chlorine pentafluoride mole fraction 00 Xarrow_forwardThe ideal gas law is a limiting law, in that it is expected to hold as pressure approaches zero. Suppose the following measured values have been obtained for oxygen gas at 273.15K. Based on the data, calculate R for each pressure value and create a table of pressure and R values (i.e. you are treating R as a variable rather than a constant at this point). Then create a plot R vs p and find the “best value” of R by determining the y-intercept of a straight line fit. (Be sure to label your axes including units).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY