
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:AH is positive
the system is endothermic
the system releases heat to the surroundings
the enthalpy of the products is greater than the enthalpy of reactants
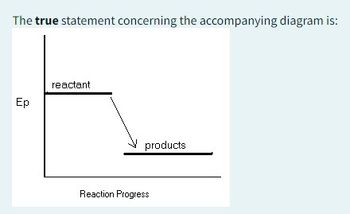
Transcribed Image Text:The true statement concerning the accompanying diagram is:
Ep
reactant
products
Reaction Progress
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- sedibias ebnerlax To 7. Writing and Balancing Reactions. Write and Balance the following reactions with the information given. A. Solid calcium reacts with oxygen to form calcium oxide. OSHS HO AS sH+s(HO)u0- OsH B. Solid sodium hypochlorate is heated, it decomposes to form solid sodiume chloride and oxygen gas. SOSH O idal olibsen teit er ni beoubon nemels ert bns besibixo Inemele eni yhitnebl A Jnemols ert bns besiblxo Inem einu ob of tnemete noss of eledmun noilsbixo C. Hydrogen sulfide gas is passed over solid hot lead (III) hydroxide, the resultant reaction produces solid lead (III) sulfide and gaseous water. ynam 1uo mont 1egaon to viearrow_forwardThe actual yield can only be determined by: 8°F Mostly cloudy O Writing a balanced equation. O Calculating the theoretical yield and plugging into the percent yield. O Reviewing past reactions. O Carrying out the reaction in a laboratory. O Search Harrow_forwardPlease send me the question in 20 minutes it's very urgent plzarrow_forward
- 20. The Haber-Bosch process, devised in the early 20th century, revolutionized global industry. It provides a way of“fixing” atmospheric nitrogen into ammonia, allowing for the production of fertilizers and the many nitrogenbased chemicals we use today. The reaction is carried out in numerous industrial facilities all over the world:arrow_forwardSilver Nitrate Solution, AgNO3 Anion Reaction With AgNO3 (ionic equations for observed reactions) Solubility in NH3 Solubility in nitric acid (HNO3) Cl- Br- I- SO32- CO32-arrow_forwardTranslate each word equation into a balanced chemical euqation, including phyical states for all reactants and products. a) silver nitrate + sodium chloride → silver chloride + sodium nitrate b) siler nitrate + calcuium chloride → sliver chloride + calcium nitrate c) hydrochoric acid + sodium hydroxide → water + sodium chloridearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY