
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
My teacher said that this has to be done using the matrix way because solving this problem is using the matrix way I was wondering if you can show me a step by step full detail using the matrix way to get an answer to solve this problem
Again this has to be done using the matrix way
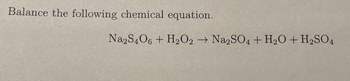
Transcribed Image Text:Balance the following chemical equation.
Na2S4O6 + H₂O2 → Na2SO4 + H₂O + H₂SO4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 11. ( Draw the potential energy as a function of x. On top of the potential function, draw horizontal lines corresponding to the ground and first two excited quantum mechanical state energies of this system. Label the lines with their respective "v labels". Finally, using your energy state lines as individual axis, draw the wave functions for the ground and first two excited states. Consider the standard one-dimensional Harmonic oscillator centered about x = 0.arrow_forward1) The following questions concern commutation relations, by applying it to an arbitrary function.arrow_forwardplease answer #5arrow_forward
- Why would CCSD and FCI give the same PES? Would you expect this result if the same calculations were performed on the water molecule? Hint: The CCSD methods includes only single and double electron excitations, whereas full CI (FCI) accounts for electron excitations from all electrons simultaneously and therefore finds the true solution of the Schrödinger equation.arrow_forwardShow that f(x) is an eigenfunction of the operator given. Thank you for the help.arrow_forwardPlease answer all in 20 minute just need answerarrow_forward
- Question: In the context of quantum chemistry, how can one accurately predict the behavior of an extremely large system of interacting molecules, considering the inherent complexity and computational limitations?arrow_forwardIs it true that the smaller the azimutal quantum number the more penetrating the orbitals? If yes/no why?arrow_forwardcan you please explainin detailarrow_forward
- Nonearrow_forwardThe Hartree-Fock self-consistent field method is an iterative approach to finding exact quantum mechanical solutions for multi-electron systems. True Falsearrow_forwardFind the simpiest Form. Determine if the operators are comuting or noncommuting 1.) ze and x 11) ê and Pxarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY