
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
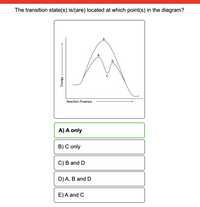
Transcribed Image Text:The transition state(s) is/(are) located at which point(s) in the diagram?
B
Reaction Progress
A) A only
B) C only
C) B and D
D) A, B and D
E)A and C
Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 18 E 20 C Submit Answer $ When H₂S(g) reacts with O₂(g) to form H₂O(g) and SO₂(g), 124 kcal of energy are evolved for each mole of H₂S(g) that reacts. 4 Write a balanced equation for the reaction with an energy term in kcal as part of the equation. Use the SMALLEST INTEGER coefficients possible and put the energy term in an appropriate box. If a box is not needed, leave it blank. 999 F4 R F V + % 5 tivity.do?locator assignment-take T G Retry Entire Group 7 more group attempts remaining tv 6 B [Review Topics] [References] Use the References to access important values if needed for this question. + MacBook Air F6 Y H TAO & 7 17 N F7 U J * - 8 30 FB M ( 9 DI K H Unline teaching and le Xx 0 V F9 I + ) 0 L command PLO F10 P A. > Y 1 option + 4 Email Instructor F11 { [ ? + Previous B + = 11 I A Next> Q2 Tue Save and Exit delete * O return shift Sarrow_forwardDetermine the total amount of heat energy, in kj, is released when 1 gallon of octane reacts in the presence of excess oxygen. (Combusts)arrow_forwardUsing the figure, answer the following questions. 3 b Re 4 Energy Reaction 5 a. Which number/letter identifies the transition state? b. which number/letter identifies the Ea of the reaction in the forward direction c. Which number/letter identifies the Ea of the reaction in the reverse direction? d. Which number/letter identifies the heat of the reaction? Write your answers in order.arrow_forward
- ANSWER WITH EXPLANATIONarrow_forwardWhen the reaction Cr2O72- + ClO2- → Cr3+ + ClO2 is balanced, what is the coefficient for ClO2 ?a) 12b) 6c) 3d) 1e) Not surearrow_forwardQUESTION 28 BrZ For the reaction to the right, a mixture of two products is obtained at both high and low temperature (though in different proportions). What are the identities of the two products? Br Br Br Br Br Br (X) CY) (2) (W) (a) W and X (b) W and Y (c) X and Y (d) W and Z O Answer (a) O Answer (b) O Answer (c) O Answer (d) QUESTION 29arrow_forward
- Part A) The standard molar heat of formation of water vapor (steam) is -242 kJ/mol. What amount of energy, in kilojoules, is produced when 1.50*108 liters of hydrogen gas at STP are combusted with excess of oxygen. (a) 1.50x108 (b) 1.62x10-9 (c) 1.62x109 (d) 8.10x108 (e) None of those. If so, what is your answer? Part B) In a reaction between nickel (II) nitrate and sodium sulfide in aqueous solution, spectator ions are: (a) sodium & sulfide (b) nickel & nitrate (c) sodium & nitrate (d) nitrate & sulfide (e) There are no spectator ions Part C) Carbon dioxide diffuses through a porous barrier at a rate of 0.200 mL/min. If the unknown gas diffuses through the same barrier and at the same conditions at a rate of 0.331 mL/min, what gas out of the following it may be? (a) Ar (b) H2 (c) NO (d) CH4…arrow_forwardNeed help with number 7 pleasearrow_forward2.6 g sodium with 5 g chlorine gas recovers 5.8 g sodium chlorine whats percent yieldarrow_forward
- Question: Consider a chemical reaction where a gaseous reactant A reacts with a solid reactant B to form a gaseous product C. The reaction is exothermic and highly endothermic. Discuss the factors that influence the reaction rate and provide an explanation for the observed energy changes during the reactionarrow_forwardQUESTION 26 The kinetic energy of a 2000.0 g bowling ball traveling at 8.0 m/s is A) 1.20 x 103 B) 64 C) 11.9 D) 1.3 x 102 E) 23.7 J. a. O earrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY