
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
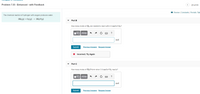
Transcribed Image Text:Problem 7.55 - Enhanced - with Feedback
23 of 33
I Review | Constants | Periodic Tab
The chemical reaction of hydrogen with oxygen produces water.
2H2(9) + O2 (g) → 2H2O(g)
Part B
How many moles of H2 are needed to react with 4.5 mol of O2 ?
?
mol
Submit
Previous Answers Request Answer
X Incorrect; Try Again
Part C
How many moles of H20 form when 3.9 mol of O2 reacts?
?
mol
Submit
Previous Answers Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please check my workarrow_forwardPlease answer question 3arrow_forwardInstructions for question: Drag a number to the blank boxes on the diagram. If the number you need is not listed, drag "number not given" to the box. Question: How many mol of NH3 are formed when 15.6 g of H2are reacted with excess N, according to the reaction below? N2 + 3 H2 → 2 NH3 mol H2 mol NH3 g H2 mol NH3 15.6 2 g H2 3 mol H2 :: 17 :: 1 :: 3 :: 5.2 :: 2.6 :: 44.2 :: number not given II ::arrow_forward
- I need help because i strugglearrow_forwardAccording to the following reaction, how many grams of nitrogen gas will be formed upon the complete reaction of 24.0 grams of ammonium nitrite? ammonium nitrite (aq) →nitrogen + water (I) grams nitrogen gas Stelry Enlire Group 7 more group attempts remaining Submit Answerarrow_forwardQuestion 4 1 pts In photosynthesis, Carbon Dioxide (CO2) and Water (H2O) go through multiple reactions to produce Glucose (CH1206) and Oxygen (O2). What is the percent yield of this reaction if 4.39 g of Carbon Dioxide only produces 0.826 g of Glucose? Remember percent yield is given as a percentage (i.e. 97%).arrow_forward
- 9 SEROol Löop If Help Synergy! SFUSD bookmarks G Image result for car... YouTube Question 3 of 5 If 74.6 g of aspirin (CsHsO4) are produced from 79.8 g of C-H&Os, what is the percent yield from the reaction below? CH.Os (s) + C&H&Os (s) → CH$O4 (s) + HC2H3O2 (aq). Type here to searcharrow_forward__H2 +__ O2 ➞ __H2O Reaction Type:___synthesis___ How many grams of water are produced from 9.25 mol hydrogen in an excess of oxygen? please show all work in answerarrow_forwardAccording to the following reaction, how many moles of chlorine gas are necessary to form 0.571 moles iron(III) chloride?iron (s) + chlorine (g) -> iron(III) chloride (s) _____ moles chlorine gasarrow_forward
- Fill in boxes with correct answers pleasearrow_forwardQuestion 16 Consider the following hypothetical unbalanced equation: D6+ Z --> DZ How many moles of the excess reactant remains when 5.5 mol of D6 reacts with 5.5 mol of Z? Please refer to the Mini Periodic Table of Fake Elements to answer your questions Mini Periodic Table of Fake Elements D J Q Z 15.00 19.00 35.00 70.00 4.58 mol 5.5 mol 0.916 mol 33 molarrow_forwardAssignment Score: 13.5% Resources O Hint Check Answer Question 19 of 26 > The combustion of ethane (C,H,) produces carbon dioxide and steam. 2C,H,(g)+70,(g) → 4 CO,(g) + 6 H,O(g) How many moles of CO, are produced when 5.10 mol of ethane is burned in an excess of oxygen? moles of CO,: mol cerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY