
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
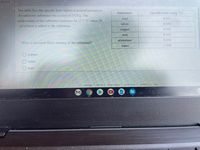
Transcribed Image Text:20 of 25.
The table lists the specific heat values of several substances.
Substance
Specific heat (cal/g. C)
An unknown substance has a mass of 11.9 g. The
8-
lead
0.031
temperature of the substance increases by 17.7 °C when 19
silver
0.058
cal of heat is added to the substance.
сopper
0.092
iron
0.107
aluminum
0.216
What is the most likely identity of the substance?
water
1.000
O copper
water
O lead
about us
careers
privacy policy
terms of use
contact us
help
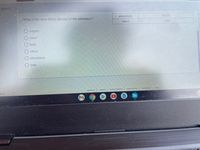
Transcribed Image Text:1 20 of 25
aluminum
0.216
What is the most likely idenity of the substance?
1.000
water
O copper
O water
O lead
O silver
aluminum
O iron
ఆండ
help
terms of use
contact us
careers
privacy policy
about us
g, Inc.
*గంపి
Σ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rachel West Section 017 A thermometer placed in a solution undergoing a chemical reaction indicates an increase in temperature as ie reaction proceeds. Is this reaction endorhermic or exothermic? Describe if heat energy is lost or gained Trom the reaction (the system) to the surroundings. What is the sign of the enthalpy change (AH) of this reaction? A student performs a reaction and determines the enthalpy change (AH) to be 31.4 kJ. Will the cemperature of the surrounding solution increase or decrease as a result of this chemical process?arrow_forward3arrow_forwardPlease help me complete this questionarrow_forward
- 3. A 74.5 g piece of metal at 86.0°C is placed in 133 g of water at 21.0°C contained in a calorimeter. The metal and water come to the same temperature at 24.2°C. How much heat (in J) did the metal give up to the water? (Assume the specific heat of water is 4.18 J/g·°C across the temperature range.) J What is the specific heat (in J/g·°C) of the metal? J/g·°C 4. A 0.528 g sample of KCl is added to 51.7 g of water in a calorimeter. If the temperature decreases by 1.07°C, what is the approximate amount of heat (in J) involved in the dissolution of the KCl, assuming the heat capacity of the resulting solution is 4.18 J/g°C? J Is the reaction exothermic or endothermic? exothermicendothermic 5. When 2.56 g of methane burns in oxygen, 128 kJ of heat is produced. What is the enthalpy of combustion (in kJ) per mole of methane under these conditions? kJ/mol methane 6. Joseph Priestly prepared oxygen in 1774 by heating red mercury(II) oxide with sunlight focused through a lens. How much…arrow_forwardA 4.81 g sample of an unknown salt (MM = 116.82 g/mol) is dissolved in 150.00 g water in a coffee cup calorimeter. Before placing the sample in the water, the temperature of the salt and water is 23.72°C. After the salt has completely dissolved, the temperature of the solution is 28.54°C. If 3.12 × 10³ J of heat was gained by the solution, what is the total heat for the dissolution reaction of the 4.81 g of salt? How many moles of the unknown salt were used in the reaction ? _______ J?arrow_forwardWhen substances are dissolved in water, they can absorb or release heat. Describe how you would use a calorimeter to determine the amount of heat required or released to when 10 grams of sugar is dissolved in 100 grams of water. Describe how you would calculate the heat absorbed or released by the sugar with the data you collect. (Do no perform any calculations.)arrow_forward
- You are studying a chemical reaction. That reaction (the system) loses heat to the surroundings. O The reaction is endothermic The reaction is exothermic Not enough information has been providedarrow_forward* 00 Σ In your own words, differentiate between an endothermic and exothermic reaction in one complete sentence. $ 4 9. Ə p altarrow_forwardA 22.43 g sample of a substance is initially at 20.1° C. After absorbing 1603 J of heat, the temperature of the substance is 171.6° C. What is the specific heat (SH) of the substance?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY