
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
ANS IS -2073.075 kJ/mol
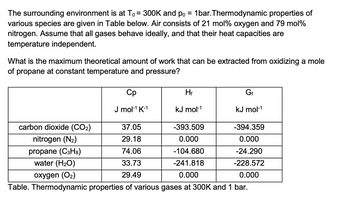
Transcribed Image Text:The surrounding environment is at T₁ = 300K and po = 1bar.Thermodynamic properties of
various species are given in Table below. Air consists of 21 mol % oxygen and 79 mol%
nitrogen. Assume that all gases behave ideally, and that their heat capacities are
temperature independent.
What is the maximum theoretical amount of work that can be extracted from oxidizing a mole
of propane at constant temperature and pressure?
carbon dioxide (CO₂)
nitrogen (N₂)
propane (C3H8)
water (H₂O)
oxygen (O₂)
Cp
J mol-¹ K-1
Hf
kJ mol-¹
Gf
kJ mol-¹
37.05
-393.509
29.18
0.000
74.06
-104.680
33.73
-241.818
29.49
0.000
Table. Thermodynamic properties of various gases at 300K and 1 bar.
-394.359
0.000
-24.290
-228.572
0.000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Similar questions
- You are asked to design a steel tank in which CO: will be stored at 300 K. The tank is 30 m: and is needed to store 1200 kg of CO. Use compressibility charts to determine the pressure exerted by the Co. :300 100 090 O 80 7090 070 100 -070 060 O 50 040 00 01 02 03 04 05 06 Reduced pressure, 07 08 09 10 4.50 45 4.00 4.0 -375 350 325 300 0.60 3.0 2.75 250 0.40 2.0 200 80 1.5 1.60 160 0.20 1.0 30 1.20 1.10 05 0.0 0.5 1.0 1.5 2.0 25 3.0 3.5 4.0 4.5 5.0 55 6.0 6.5 7.0 Retuced pressare, P. 19 Compressibility foclor, Zpv/RT - - oe non-undarrow_forwarda - (P-+-)V=K A fluid follows the following equation of state: V=RT where a is a constant that depends on the critical properties of the fluid. Calculate the Residual Gibbs Free Energy of this fluid at T-30 °C and P=10 bar, if the compressibility factor at these conditions is Z=0.88. Express your result in kJ/kmol and round your numerical answer to whole number.arrow_forwardChemical Engineering A tank contains 1 kg mass gas whose density is 700 kg/m3. The pressure is increased from 1 bar to 3 bar. The approximate specific boundary work of the system is Cannot be find since some data is missing 285 kJ/kg 0 kJ/kg 0.285 kJ/kgarrow_forward
- A physics experiment is conducted at a pressure of 14.0 kPa. What is this pressure in mmHg? 105 mmHg 18.4 mmHg O1.87 mmHg O1.40 x 10ª mmHg O1.84 x 102 mmHgarrow_forwardCalculate the melting point of ice under a pressure of 10 MPa in Kelvin. Assume that the density of ice under these conditions is approximately 0.915g/cm and that of liquid water is 0.998 g/cm?.arrow_forwardAir expands adiabatically in a piston-cylinder assembly from an initial state where p₁ = 100 lbf/in.², v₁ = 3.704 ft³/lb, and T₁ = 1000 °R, to a final state where p₂ = 30 lbf/in.² The process is polytropic with n = 1.4. The change in specific internal energy, in Btu/lb, can be expressed in terms of temperature change as Au = (0.171)(T2-T1). Determine the final temperature, in °R. Kinetic and potential energy effects can be neglected. T₂ = 1 °Rarrow_forward
- For chemical Engineers. The specific volume of wet steam at 500 kpa is 0.2813 m3/kg. using the data from steam tables, determine the quality of steamarrow_forwardQ1) A steel cylinder 10 cm in diameter and 10 cm long is initially at 300 °C. It is suddenly immersed in an oil bath that is maintained at 40 °C, with h =280 W/m² °C. Find: (a) The temperature at the center of the solid after 2 min, and (b) The temperature at the center of one of the regular faces after 2 min. Answers a) T = 175 °C b) T = 151 °Carrow_forwardOne mole of gas in a closed system undergoes a four-step thermodynamic cycle. Use the data given in the following table to determine numerical values for the missing quantities indicated by question marks. Step AU/J QIJ W/J 12 -200 ? -6000 23 -3800 ? 34 ? -800 300 41 4700 ? 12341 ? ? -1400arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The