
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
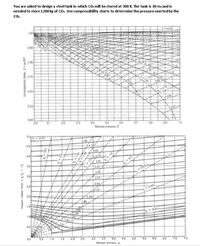
Transcribed Image Text:You are asked to design a steel tank in which CO: will be stored at 300 K. The tank is 30 m: and is
needed to store 1200 kg of CO. Use compressibility charts to determine the pressure exerted by the
Co.
:300
100
090
O 80
7090
070
100
-070
060
O 50
040
00
01 02
03
04
05
06
Reduced pressure,
07
08
09
10
4.50
45
4.00
4.0
-375
350
325
300
0.60
3.0
2.75
250
0.40
2.0
200
80
1.5
1.60
160
0.20
1.0
30
1.20
1.10
05
0.0
0.5
1.0
1.5
2.0
25
3.0
3.5
4.0
4.5
5.0
55
6.0
6.5
7.0
Retuced pressare, P.
19
Compressibility foclor, Zpv/RT
- - oe non-und
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 1. It is proposed to pump 10,000 kg/h of toluene at 114 °C and 1.1 atm absolute pressure from the reboiler of a distillation tower to a second distillation unit without cooling the toluene before it enters the pump. If the frictional loss in the line between the reboiler and pump is 7 kN/m2 and the density of toluene is 866 kg/m3 , how far above the pump must the liquid level in the reboiler be maintained to give a net positive suction head of 2.5 m? The answer should be: 3.22 m 2. 2.A pump moves hot water condensate from a tank under vacuum (T = 180°F and p = ‐9.8 psig) to a vented tank at an elevation 40 ft above the pump. The condensate level is 5 ft above the pump inlet, the vapor pressure of the condensate is 7 psia, and the head loss in the suction line is 2.3 ft. If the minimum required NPSH is 9 ft, will the pump cavitate? (Please type answer no write by hend)arrow_forwardIf I have gas at 1.0 atm of pressure and 30oC, what will I have to raise the temperature to in order to get 6.50 atm of pressure?arrow_forwardTo find CP/CV of a gas, one sometimes uses the following method. A certain amount of gas with initial temperature T0, pressure P0, and volume V0 is heated by a current through a platinum wire for a time t. The experiment is done twice: first with a constant volume V0 with pressure changing from P0 to P1, and then at a constant pressure P0 with the volume changing from V0 to V1. The time t is the same in both experiments. Find the ratio CP/CV. The gas may be considered ideal.arrow_forward
- Calculate the melting point of ice under a pressure of 10 MPa in Kelvin. Assume that the density of ice under these conditions is approximately 0.915g/cm and that of liquid water is 0.998 g/cm?.arrow_forward1. Consider a 1000 MW power plant located in a rural area with 15 ton/day SO2 emissions from a 100 m high stack. The velocity and temperature of the stack gases lead to an effective stack height of 50 m above the physical stack. Estimate the ground level concentration as a function of distance downwind under the following conditions. The emissions are into a clear daytime atmosphere with wind (at 10 m) of 5 m/s. b. The emissions are into a clear nighttime atmosphere with wind (at 10 m) of 2 m/s. The conditions of a. except there is a strong elevated inversion at an altitude of 200 m. a. С.arrow_forwardchemical engineering A power plant emits 216 g of SO2 every hour. The wind recorded on the same day is 5 m s-1, and the atmospheric stability class is A for the clear summer afternoon. There is no plume rise, and the stack height is 30 m. The values of σy and σz are 215 m and 450 m, respectively. Determine: the concentration of SO2 0.7 km downwind along the plume centreline at ground level.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The