
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
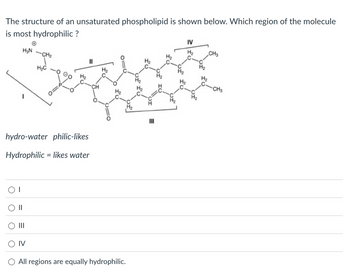
Transcribed Image Text:The structure of an unsaturated phospholipid is shown below. Which region of the molecule
is most hydrophilic ?
H₂N-CH₂
H₂C
IV
CH3
CH3
hydro-water philic-likes
=
Hydrophilic likes water
○ IV
All regions are equally hydrophilic.
III
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- 10 Jlgw What are the functional groups present in Aspirin which has the following structural formula aule ulao ut الدرجة من 1 C-OH lio ple P السؤال 0-C-CH3 alcohol , ketone and ether .a O carboxylic acid and ester .b O carboxylic acid , ether and ketone .c C aldehyde and ketone .dOarrow_forwardAnsaid and Motrin belong to the group of drugs known as nonsteroidal anti-inflammatory drugs (NSAIDs). Both are only slightly soluble in water, but one is a little more soluble than the other. Which of the drugs has the greater solubility in water?arrow_forwardWrite the balanced equation for the following reaction, Oxidation of Cyclohexanol with Hypochloritearrow_forward
- 4. Predict the esters formed in the following reactions. A catalytic amount of acid is added in each reaction. HO H3C + H;C HO, H3C HO, I OH H2 HO H3C HOarrow_forwardConsider a molecule of methyl methacrylate, shown above. If this molecule were to engage in hydrogen bonding with a molecule of acetone cyanohydrin, which would be the hydrogen bond acceptor and which would be the hydrogen bond donor?arrow_forwardIn which of the following alcohol solvents do you think NaCl would be the most soluble? CH;OH CH;CH2OH CH3CH,CH,OH CH;CH,CH,CH,OH A В Darrow_forward
- Give detailed Solution with explanation (no need Handwritten answerarrow_forwardaw the products for the following reactions showing the formation of amides. Name the 5. reactants and the products. CH Nit + Ho- Cat b) CH-CH NH looc Cat. oH -WH looc cat 6. Draw the products when each of the amides undergoes acid hydrolysis. Name the reactants and products. HCL Cty- -NH-C_Cy+ 0 HCL b) C-CH-N-&cnycy +4,0 HCL + H,0 digarrow_forwardHO HO. OH base CI EIO- C-OEt epichlorohydrin OH NaOEt 2,6-dihydroxy- acetophenone *Na O2C. .CO2 Na* NaOH, H2O II OH Cromolyn sodium Cromolyn sodium, developed during the 1960s, has been used to prevent allergic reactions primarily affecting the lungs, as, for example, exercised- induced emphysema. It is thought to block the release of histamine, which prevents the sequence of events leading to swelling, itching, and constriction of bronchial tubes. v Cromolyn sodium is synthesized in the above series of steps. rch 31% 11:24 P 4/20/20-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
