
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
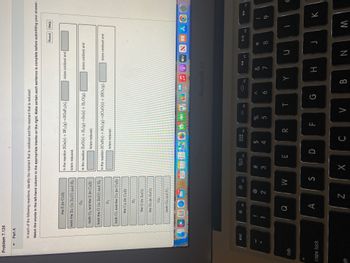
Transcribed Image Text:Problem 7.124
ift
tab
caps lock
Part A
In each of the following reactions, identify the reactant that is oxidized and the reactant that is reduced:
Match the words in the left-hand column to the appropriate blanks on the right. Make certain each sentence is complete before submitting your answer.
esc
the S (In CuS)
both the Zn (in ZnO) and H₂
0₂
both O₂ and the S (in CuS)
both the O (in ZnO) and H₂
F₂
both O₂ and the Cu (in CuS)
the Cu (in CuS)
1
the O (in ZnO)
the Zn (in ZnO)
H₂
F1
both Ga and F₂
Q
Ga
A
2
Z
F2
W
In the reaction 2Ga(s) + 3F2 (g)-2GaF3 (s),
is/are reduced.
JUL
27 6
S
In the reaction ZnO(s) + H₂(g)→Zn(s) + H₂O(g),
is/are reduced.
In the reaction 2CuS(s) + 302 (g)→2CuO (s) + 2SO2 (g).
is/are reduced.
ㅁㅁ
3
X
F3
E
D
54
00
C
F4
R
F
%
5
F5
T
V
G
is/are oxidized and
MacBook Air
F6
Y
is/are oxidized and
B
7
H
is/are oxidized and
tv
F7
U
Reset Help
N
8
J
A
F8
1
O
M
K
F9
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- coz + PbL NO3)z C03? + Pb2 +(Uo;) = Pb CO3 + NO 2) SO4 + Ba (NO;)2 So4 + Ba+ (U0s) → Ba(sou) + NOz + balNOs)z 2- 5) CrOa* + Fe(NO,) 6) I + Fe (NO,) 3 D CI + Ag NO3 8) NO + Ag WOsarrow_forwardWhat is the charge for the transition metal Manganese in Manganese (III) oxide? A.+1 B.+2 C.+3 D.+4arrow_forwardRe-order each list in the table below, if necessary, so that the atoms or ions in it are listed in order of decreasing size. atoms or ions 2- Se, Se, Br F, CI, CI S, Al, P Explanation atoms or ions in order of decreasing size 0.0.0 0.0.0 0.0.0 Check X 00 G Ⓒ2022 MCGIDw Mill LLC. All Rights Reserved. Terms of Usearrow_forward
- Hi could someone help me with this question. Thank you!arrow_forwardme File Edit View History Bookmarks People Tab Window Help HCc Dashboar x E Buy Essay x 5% O G find some x © Periodic A ALEKS - J X HUc Chapter 5 x G convert C A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQİHQRDYV_6Ux63SypJXz0Coxvwqgg4JkWI74Meq7fCi1Pj.. O GASES Search Calculating partial pressure in a gas mixture S jacquehr mail.goo Jacqueline Profile - web.robl Cuteding of the mi A 9.00 L tank at 3.36 °C is filled with 13.2 g of boron trifluoride gas and 9.61 g of dinitrogen difluoride gas. You can assume both gases behave as ideal gases under these conditions. R Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. Virtual E R - Roblox web.robl Virtual B group on mole fraction: dh boron trifluoride Manage appleid.a Your App partial pressure: | atm le for account mole fraction: dinitrogen diffluoride ble to partial pressure: ||atm Total pressure in tank: | atm ranscrip…arrow_forwardThe element which has four valence electrons is ________.( A) H B) Na C) Mg D) Si E) Sarrow_forward
- Which of the element listed below does not produce any color on flame test? * Cs Rb Mg O Sr Which of the element listed below does not produce any distinct color on flame test? (flame test of the element produces white sparks only) * Cs Rb O Mg Sr A chemical element previously used to produce blue color in fireworks but was later replaced due to environment and health hazard. * Cu As Pb Веarrow_forwardPronies lab Window Help Workshop X PB 11.3 Pers X DZL Homepag X ent/takeCovalentActivity.do?locator=assignment-take ap X docx 1A H2A Li Be A Na Mg 38 48 58 68 7888 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Fr Ra Rc Rf Ha *** Submit Answer Akon - Be X 3A 4A 5A 6A 7A He BCNO F Ne 18 2B Al Si P S Cl Ar Ga Ge As Se Br Kr w- Workshop.docx NOV 28 tv (1) What is the element with an electron configuration of 1s22s22p63s²3p64s23d¹? In Sn Sb Te I Xe Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr (2) What is the element with an electron configuration of 1s²22s22p63s23p64s¹? Q 8A Retry Entire Group 9 more group attempts remaining Pb Bi Po At Rn .l C From the X A P OWLv2 | X Previous W Oarrow_forward116 C,HO, =122.12 g/mol 2C,H,O, + 150, - 14CO, + 6H,0 What mass of CO, is released when 6.00 moles of C,HO, are added? Show work here eaker notesarrow_forward
- Plz use the given below periodic table to write the formula for the compound 1- bellium tigerite 2- mollium callide 3-romeomium (II) smokite 4- dasium hydrogen calliite 5- jackium (II) kittite And for this part also use the below given periodic table to write the name for the formula 1- Mo3L2 2- Ro(ScO3)3 3- J(LuO4)2 4-Mi2O3 5- Mi2O 6- HScO3 I know you guys only solve up to three sub parts but plz solve it for me... I really need it... May you have the best day of your life... God bless youarrow_forwardCHEMISTRY Use the Aluminu Key aluminur 11- Na -Eemont symbol Sodium- Element name Atomic number 22.99 Average atomic mass* A anganer Ir 192 25 105 MI Db Sg Meitnan (261) Ce Sm Dy Ho Pr *is number ion parantheses, then t refors to the atomic mass of the most stable isoope. 150 30 182 50 Th Pa Pu Cf Es 232.01 231.04 238 03 品 1p32 品 8あa 0 8>arrow_forward4. Which of the following shows a gain of electrons? a) Ca2*(aq) to Ca(s) b) 2Cl(aq) to Cl2(9) c) Fe2*(aq) to Fe3*(aq) d) NaCl(s) to Na*(aq) and Cl(aa)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY