
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
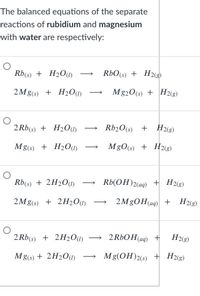
Transcribed Image Text:The balanced equations of the separate
reactions of rubidium and magnesium
with water are respectively:
Rb(s) + H2O1)
RbO(s)
+ H2(g)
2Mg(s) + H20(1)
Mg20(s) + H2(g)
2 Rb(s) + H2O(1)
Rb20(s) +
H2(g)
Mg(s) + H2O(1)
MgO(s) + H2(g)
Rb(s) + 2H2O(1)
Rb(OH)2(aq) + H2«3)
2Mg(s) + 2H2O(1)
2M8OH(aq) +
H2(g)
2 Rb(s) + 2H201)
2 RbO H(aq) +
H2(g)
Mg(s) + 2H2O(I)
Mg(OH)2(s) + H2«g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance these chemical equations. (Use the lowest possible whole number coefficients.) (a) S8 + O2 → SO3 chemPad Help (b) NaNO3 → NaNO2 + O2 chemPad Help (c) C4H10 + O2 → CO2 + H2O chemPad Help (d) AgCl2 + H2 → Ag + HCl chemPad Help (e) Ga + H2SO4 → Ga2(SO4)3 + H2 chemPad Help (f) N2H4 + O2 → H2O2 + N2 chemPad Helparrow_forwardBalancing Chemical Equations Cu(s) O(g) → CuO(s) 28 Al+ H,SO. H + H0(1) → H(9) + 29 Si,H O, > SIO, HO Fe(s) H,O(g) → H:(g) Fe,O.(s) 30 NH, + H,O ASCI(aq) H;S(aq) → AS S(s) + HCI(aq) co, + 31. CuSO. 5H,0(s) → CuSO(s) + H;0(g) 32 BN + BF, + Fe Os(s) Hg) + Fe(S) +_H;O(1) 33. CaSO,+2H,0 + 50, → Caso, HSO. CaCO,(s) → CaO(s) + Co.(g) 34 C,H 0, CO, FeS(s) 35. C,H,0, 0. > CO, + • (s)3 HS(04) S(s) > HOH(I) + KS(aq) ZnI, > Nal Zn KOHaq) → 36 Na + 10. Na(1) + CI,(g) 37. HBr0, HBr > H,O Br. 11 Al(S) H,S0.(aq) + H(g) * Al,(SO,), 38 AI,C, + CH. NH,OHaq) + HOH(I) (NH.),PO,(aq) (beodH Ca(NO,),-3H,0 + LaC, Ca(NO,), La(OH CH 39 12. 13. CHIQ) CO.(9) H,0(1) 40. CH,NO, Cl, + CCI,NO, = HC Ca,(PO.), SiO, CaSiO, CO P 41. 14 AS) 0,9) + (s) o'iv CHE H,0(1) Al,C. + HO + AI(OH 42 15 CHdg) Co(g) * 43 Naf Cao + Caf NOOH 16 KINO, KNO, 44 AICI,> LIAIH. LICI 17 Coc Ca + Co, Caf,+ SiO, క్రచరి 18 CH + CO, + HO 45 HSO. 46 Casi, + SbCl, > CoCl 19. KSO KCI BaSO. TiO, B.C+ TiB CO 47 20 KOH + H,50. KSO. 48. NH, + NO HO 21…arrow_forwardCryolite, Na3AlF6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. Balance the equation. 1.) Balance the equation - AlO3(s)+NaOH(l)+HF(g)-->Na3AlF6+H2O(g) 2.)If 16.4 kilograms of Al2O3(s), 60.4 kilograms of NaOH(l), and 60.4 kilograms of HF(g) react completely, how many kilograms of cryolite will be produced? 3.)Which reactants will be in excess, (Al2O3, NaOH, or HF) 4.)What is the total mass of the excess reactants left over after the reaction is complete in KG?arrow_forward
- Fill in the blanks using the other image.arrow_forwardIn the replacement reaction of magnesium nitrate with tin, how many grams of tin(IV) nitrate would be produced if 85.2 mg of magnesium nitrate? Report your answer with 3 significant figures. 2 Mg(NO3)2 + Sn → Sn(NO3)4 + 2 Mgarrow_forwardBalance the following equations: (a) Ag(s)+H2S(g)+O2(g)⟶Ag2S(s)+H2O(l)Ag(s)+H2S(g)+O2(g)⟶Ag2S(s)+H2O(l) (b) P4(s)+O2(g)⟶P4O10(s)P4(s)+O2(g)⟶P4O10(s) (c) Pb(s)+H2O(l)+O2(g)⟶Pb(OH)2(s)Pb(s)+H2O(l)+O2(g)⟶Pb(OH)2(s) (d) Fe(s)+H2O(l)⟶Fe3O4(s)+H2(g)arrow_forward
- Sodium hydroxide reacts with phosphoric acid to yield sodium phosphate and water. If 45 g of NaOH reacts with 35 g of H3PO4, what is the limiting reagent of this reaction if it yields a certain amount of NA3PO4? (Atomic masses: Na = 22.99 u ; O = 16.00 u; H = 1.01 u ; P = 30.97 u) Phosphoric Acid Sodium Hydroxide Water Sodium Phosphate 00arrow_forwardIn the replacement reaction of magnesium nitrate with tin, how many grams of tin(IV) nitrate would be produced if 89.5 mg of magnesium nitrate? Report your answer with 3 significant figures. 2 Mg(NO3)2 + Sn → Sn(NO3)4 + 2 Mgarrow_forwardCryolite, Na3AlF6(s),Na3AlF6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. Balance the equation for the synthesis of cryolite. equation: Al2O3(s)+NaOH(l)+HF(g)⟶Na3AlF6+H2O(g)Al2O3(s)+NaOH(l)+HF(g)⟶Na3AlF6+H2O(g) If 15.2 kg of Al2O3(s),15.2 kg of Al2O3(s), 56.4 kg of NaOH(l),56.4 kg of NaOH(l), and 56.4 kg of HF(g)56.4 kg of HF(g) react completely, how many kilograms of cryolite will be produced? mass of cryolite produced: Which reactants will be in excess? a. Al2O3Al2O3 b. NaOHNaOH c. HF What is the total mass of the excess reactants left over after the reaction is complete? total mass of excess reactants:arrow_forward
- What mass of Cu(IO3)2 can be formed from 0.650 g of CuSO4 · 5H2O? What mass of KIO3 is needed to convert the copper in 0.2750 g of CUSO4 - 5H2O to Cu(IO3)2?arrow_forwardBalance these chemical equations. (Use the lowest possible whole number coefficients.) (a) C6H5OH + O2 → CO2 + H2O (b) AgCl2 + H2 → Ag + HCl (c) CuCl + H2 → Cu + HCl (d) Ga + H2SO4 → Ga2(SO4)3 + H2 (e) K + Br2 → KBr(f) NaNO3 → NaNO2 + O2arrow_forwardThe number of moles of iron produced from 0.216 moles of aluminum is: 3 FeO (1) + 2 AI (I) ---> 3 Fe (I) + 1 Al2O3 (S)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY