
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:O Question 13 - 101 final exam - Cc X
ducation.com/ext/map/index.html?_con3con&external_browser=D0&launchUrl=https%253A%252F%252Flms.mheducation.com%252Fr
am
Saved
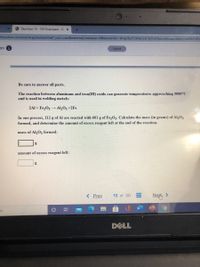
Be sure to answer all parts.
The reaction between aluminum and iron(III) oxide can generate temperatures approaching 3000°C
and is used in welding metals:
2Al + FezO3 Al2O3 +2Fe
In one process, l12 g of Al are reacted with 601 g of Fe,O3. Calculate the mass (in grams) of Al,O3
formed, and determine the amount of excess reagent left at the end of the reaction.
mass of Al,0, formed:
g
amount of excess reagent left:
< Prev
13 of 30
Ma >
ch
DELL
近
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write a balanced chemical equation based on the following description: solid tetraphosphorus (P₄) reacts with oxygen gas to produce solid tetraphosphorus decoxide. P4(s)+O2(g)_arrow_forwardOne of the reactions in the Solvay Process involves the reaction of calcium oxide with water toproduce slaked lime (calcium hydroxide).CaO(s) + H O2 (l) - Ca(OH)2 (s)A lab technician performs the experiment and uses 54.8 g of calcium oxide to react with a setamount of water. When the reaction is completed, the technician uses a filtration apparatus tocollect the mass of calcium hydroxide produced. The following data is recorded:Mass of filter paper: 1.24 gMass of filter paper and solid: 68.04 g What is the theoretical mass of calcium hydroxide that is predicted to be produced? What mass of precipitate was collected in this experiment? What is the percent error? Comment on the validity of the resultsarrow_forwardMethanol has tremendous potential to be used as an alternate fuel. Carbon monoxide gas reacted with hydrogen gas can be used to produce methanol. A student reacted 32.9 g of H2(9) with excess CO(9) to produce methanol. If his percent yield was 47.7%, calculate the mass (in g) of CH3OH(1) that he collected in the experiment. Hint: Write a balanced chemical reaction equation. Do not show your work in the space provided. Record only your final answer with the correct number of significant digits and the proper units.arrow_forward
- This question has two parts and requires two answers. Consider the reaction 4 Fe + 3 02→ 2 Fe203 a) What is the theoretical yield, in grams, of iron(III) oxide if 20.0 g of iron are mixed with 10.5 grams of oxygen gas? b) What is the limiting reactant?arrow_forwardThe theoretical yield of reactions Consider the reaction 1CO(g) + O2 (g) --> 2CO2(g) if 9.420 g CO is mixed with 16.84g O2, CALCULATE THE THEORETICAL YIELD (g) of CO2 produced by the reaction.arrow_forwardBalance the following chemical equation (if necessary) for the combustion reaction of propane C3H8(g)+O2(g)_CO2(g)+H2O(g)arrow_forward
- What mass of CO2 is produced from the combustion of 1 gal of gasoline? The chemical formula of gasoline can be approximated as C8H18. Assume that there are 2801g of gasoline per gallon.arrow_forwardComplete combustion of 4.5892 a particular compound that contains only carbon, hydrogen and oxygen produces 10.4324 g of CO2 and 4.2705 g of H2O. The empirical formula of the compound is given by C H O (indicate "1" if there is only one atom of a given type in the empirical formula.) The molar mass of the compound is found to be 116.16 g/mol in a different experiment. What is the molecular formula of the compound? C H Oarrow_forwardWrite a balanced chemical equation based on the following description: butane gas reacts with oxygen gas to produce carbon dioxide gas and water vapor C4H10(g)+O2(g)_arrow_forward
- Consider the following chemical equation: NH4NO3 (s) → N2O (g) + H2O (g) What would be the coefficients in front of each reactant and product in the balanced equation? If there would be no coefficient in front of a given compound in the balanced equation, just write type '1' NH4NO3 =? N2O =? H2O =?arrow_forwardLead ions can be precipitated from solution with KCl according to the reaction: Pb2+ (aq) + 2KCl(aq) → PbCl₂ (s) + 2K+ (aq) When 34.3 g KCl is added to a solution containing 25.8 g Pb²+, PbCl2 (s) forms. The solid is filtered and dried and found to have a mass of 30.9 g. theoretical yield of PbCl2 = 25.8 g Ph Part C % yield = Determine the percent yield for the reaction. Express your answer in percent to three significant figures. 15 ΑΣΦ X Ċ 1 mel P5²+ 1 mol 1 met X 207.2 g Pb² ? %arrow_forwardFor the following reaction, 37.3 grams of sulfuric acid are allowed to react with 42.5 grams of zinc hydroxide. sulfuric acid (aq) + zinc hydroxide(s) → zinc sulfate (aq) + water (1) What is the maximum amount of zinc sulfate that can be formed? Mass= What is the formula for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? Mass= g.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY