
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
![### Precipitation of Lead Ions
Lead ions can be precipitated from a solution with KCl according to the reaction:
\[ \text{Pb}^{2+} \text{(aq)} + 2\text{KCl(aq)} \rightarrow \text{PbCl}_2 \text{(s)} + 2\text{K}^+ \text{(aq)} \]
### Reaction Details
When 34.3 g of KCl is added to a solution containing 25.8 g of \(\text{Pb}^{2+}\), PbCl₂(s) forms. The solid is filtered and dried, and it is found to have a mass of 30.9 g.
### Calculating Percent Yield
**Part C: Determine the percent yield for the reaction.**
Express your answer in percent to three significant figures.
#### Formula for Percent Yield
\[ \text{Percent Yield} = \left( \frac{\text{Actual Yield}}{\text{Theoretical Yield}} \right) \times 100 \]
#### Given Data
- Actual Yield: 30.9 g
- **Theoretical Yield** (provided above): 25.8 g
**Calculation:**
1. **Identify the masses of reagents and products:**
- Actual yield of PbCl₂(s): 30.9 g
- Theoretical yield of PbCl₂(s): 25.8 g
2. **Determine the percent yield:**
\[
\text{Percent Yield} = \left( \frac{30.9 \, \text{g}}{25.8 \, \text{g}} \right) \times 100 \approx 119.77\%
\]
3. **Round to three significant figures:**
\[
\text{Percent Yield} \approx 119.8\%
\]
**Inputs for Calculation:**
\[
\text{\% yield} = \boxed{ \quad } \%
\]](https://content.bartleby.com/qna-images/question/6ae95cc5-a0cd-43b0-8331-9678c9dacca0/a26dddea-a9d5-42c5-862e-8a1067b86e42/hzcevt.jpeg)
Transcribed Image Text:### Precipitation of Lead Ions
Lead ions can be precipitated from a solution with KCl according to the reaction:
\[ \text{Pb}^{2+} \text{(aq)} + 2\text{KCl(aq)} \rightarrow \text{PbCl}_2 \text{(s)} + 2\text{K}^+ \text{(aq)} \]
### Reaction Details
When 34.3 g of KCl is added to a solution containing 25.8 g of \(\text{Pb}^{2+}\), PbCl₂(s) forms. The solid is filtered and dried, and it is found to have a mass of 30.9 g.
### Calculating Percent Yield
**Part C: Determine the percent yield for the reaction.**
Express your answer in percent to three significant figures.
#### Formula for Percent Yield
\[ \text{Percent Yield} = \left( \frac{\text{Actual Yield}}{\text{Theoretical Yield}} \right) \times 100 \]
#### Given Data
- Actual Yield: 30.9 g
- **Theoretical Yield** (provided above): 25.8 g
**Calculation:**
1. **Identify the masses of reagents and products:**
- Actual yield of PbCl₂(s): 30.9 g
- Theoretical yield of PbCl₂(s): 25.8 g
2. **Determine the percent yield:**
\[
\text{Percent Yield} = \left( \frac{30.9 \, \text{g}}{25.8 \, \text{g}} \right) \times 100 \approx 119.77\%
\]
3. **Round to three significant figures:**
\[
\text{Percent Yield} \approx 119.8\%
\]
**Inputs for Calculation:**
\[
\text{\% yield} = \boxed{ \quad } \%
\]
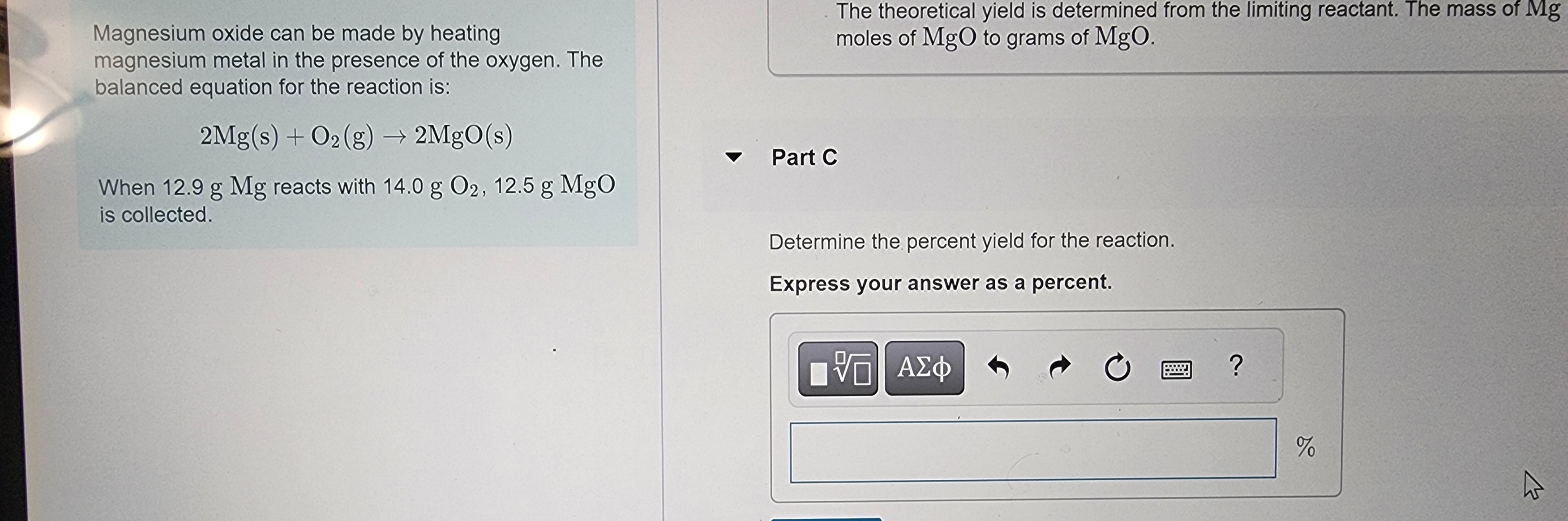
Transcribed Image Text:Magnesium oxide can be made by heating
magnesium metal in the presence of the oxygen. The
balanced equation for the reaction is:
2Mg(s) + O2(g) → 2MgO(s)
When 12.9 g Mg reacts with 14.0 g O2, 12.5 g MgO
is collected.
The theoretical yield is determined from the limiting reactant. The mass of Mg
moles of MgO to grams of MgO.
- Part C
Determine the percent yield for the reaction.
Express your answer as a percent.
[ΨΕΙ ΑΣΦ
Ú
?
%
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Sulfuric acid dissolves aluminum metal according to the following reaction: 2Al(s)+3H 2SO4(aq)→Al2(SO4)3(aq)+3H2(g) Suppose you wanted to dissolve an aluminum block with a mass of 14.8 gg . What mass of H2 gas would be produced by the complete reaction of the aluminum block?arrow_forwardA solution of NaCl(aq) is added slowly to a solution of lead nitrate, Pb(NO3)₂(aq), until no further precipitation occurs. The precipitate is collected by filtration, dried, and weighed. A total of 18.46 g PbCl₂ (s) is obtained from 200.0 mL of the original solution. Calculate the molarity of the Pb(NO3)₂(aq) solution. concentration: M Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemistry | Publisher: University Science Booksarrow_forwardExplain why the solid precipitate Agl (s) forms when solutions of KCl (aq) and AgNO3 (aq) are mixedarrow_forward
- Classify each chemical reaction: Reaction Zn(s) + FeSO₂ (aq) → ZnSO₂ (aq) + Fe(s) CuSO₂ (aq) + ZnCrO₂ (aq) → ZnSO₂ (aq) + CuCrO₂ (s) - 2 Na(s) + Cl₂(g) → 2NaCl(s) 2H₂O₂(1)→ 2H₂O() + 0₂ (8) Type ✓ choose one combination decomposition single substitution double displacement none of the above choose onearrow_forwardConsider the following reaction H2S (g) + FeS(s) + CO2(g) →FeS2(s) + HCO2H(aq) If 2.00 g of each reactant is each dissolved in 250.0 mL of water and all the solutions are mixed together. The final volume was measured to be 750.0 mL. a)How many grams of CO2 left unreacted? b) What is the concentration of HCO2H in this solution?arrow_forwardClassify each chemical reaction: KOH(aq) + HBrO (aq) reaction K BrO (aq) + H₂O (1) Na Cl (aq) + AgNO3(aq) → NaNO3(aq) + Ag Cl (s) 16K (s) + S₂ (s)→ 8K₂S (s) 2CH₂CH₂CO₂H (1) + 70₂(g) → 6CO₂(g) + 6H₂O(g) ✓ 6 ✓ 7 type of reaction (check all that apply) combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition ✓8 X precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base 9 10arrow_forward
- How many grams of Cu(OH)2 will precipitate when excess KOH solution is added to 41.0 mL of 0.618 M CuBr2 solution? CuBr2(aq) + 2KOH(aq) Cu(OH)2(s) + 2KBr(aq)g?arrow_forwardHow many grams of Ag₂CO3 will precipitate when excess Na₂CO3 solution is added to 49.0 mL of 0.590 M AgNO3 solution? 2AgNO3(aq) + Na2CO3(aq) → Ag2 CO3(s) + 2NaNO3(aq) garrow_forwardA solution of NaCl(aq) is added slowly to a solution of lead nitrate, Pb(NO3)₂(aq), until no further precipitation occurs. The precipitate is collected by filtration, dried, and weighed. A total of 10.67 g PbCl₂ (s) is obtained from 200.0 mL of the original solution. Calculate the molarity of the Pb(NO3)₂(aq) solution. concentration: Marrow_forward
- One way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate, solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 200. mL sample of groundwater known to be contaminated with cadmium chloride, which would react with silver nitrate solution like this: CdCl2(aq) + 2 AgNO3(aq) → 2 AgCl(s) + Cd (NO3)2(aq) The chemist adds 89.0 mM silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he has collected 5.4 mg of silver chloride. Calculate the concentration of cadmium chloride contaminant in the original groundwater sample. Be sure your answer has the correct number of significant digits. E d OL Earrow_forward250 mL of a 1.9 M Mg(OH)2 solution is added to a beaker. How many moles of Mg(OH)2 were added? How many grams of Mg(OH)2 were added?arrow_forwardThe balanced chemical equation for the reaction between calcium hydroxide and hydrochloric acid is: Ca(OH)2 ( aq) + 2 HC1 ( aq )→ CaCl2 ( aq ) + 2 H,0 (1) We can interpret this to mean: 1 mole of calcium hydroxide and |mole(s) of hydrochloric acid React to produce: | mole(s) of calcium chloride and |mole(s) of waterarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY