
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
infoPractice Pack
Question
infoPractice Pack
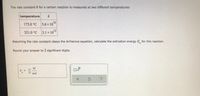
Transcribed Image Text:The rate constant k for a certain reaction is measured at two different temperatures:
temperature
173.0 °C 5.6x 1010
321.0 °C 3.1 x 1o12
Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E for this reaction.
Round your answer to 2 significant digits.
kJ
E
mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Includes step-by-step video
Learn your wayIncludes step-by-step video
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The rate constant k for a certain reaction is measured at two different temperatures: temperature k 64.0 °C 9.9 × 1012 195.0 °C 9.8 × 1014 Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E, for this reaction. a Round your answer to 2 significant digits.arrow_forwardThe rate constant k for a certain reaction is measured at two different temperatures: temperature k 354.0 °C 3.9 x 10° 233.0 °C 1.5 x 10 Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E, for this reaction. a Round your answer to 2 significant digits. kJ E x10 a mol ?arrow_forwardConsider the following general reaction for which gases A and B are mixed in a constant volume container: A(g) + B(g) -> C(g) + D(g) Match what happens to the rate of the reaction under the following changes: (consider each change separately) v all of gas B is removed from the container more gas A is added to the container the temperature of the container is increased there is no change to the reaction rate ya catalyst is added to the container I. the reaction proceeds at a faster rate v gas D is also added to the container the reaction proceeds at a slower rate II. some of gas B is removed from the container Iy the reaction does not proceed at all the volume of the container is increasedarrow_forward
- The rate constant k for a certain reaction is measured at two different temperatures: temperature k 398.0 °C 8.9 x 10' 262.0 °C 8.0 x 106 Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E for this reaction. Round your answer to 2 significant digits. kJ E = x10 mol X S C.arrow_forwardThe rate constant k for a certain reaction is measured at two different temperatures: temperature k 330.0 °C 3.6 × 10 264.0 °C 1.3 × 10* Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E, for this reaction. a Round your answer to 2 significant digits. kJ E a molarrow_forwardThe rate constant k for a certain reaction is measured at two different temperatures: temperature k 314.0 °C 2.1 x 10¹1 384.0 °C 4.3 × 10¹1 Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E for this reaction. a Round your answer to 2 significant digits. kJ E = x10 a mol X Ś ?arrow_forward
- The rate constant k for a certain reaction is measured at two different temperatures: temperature k 59.0 °C 6.5 x 101 14 163.0 °C |4.2 × 10 Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E, for this reaction. a Round your answer to 2 significant digits. kJ E x10 molarrow_forwardThe rate constant for the formation of hydrogen iodide from the elements H₂(g) + 1₂(g) → 2Hİ(g) is 2.7 x 10-4 L/(mol-s) at 600 K and 3.5 x 10-3 L/(mol·s) at 650 K. a. Find the activation energy Ea. J/mol b. Then calculate the rate constant at 719 K. L/(mol.s)arrow_forwardThe rate constant k for a certain reaction is measured at two different temperatures: temperature k 61.0 °C 6.7 x 10° -76.0 °C 4.5 x 10* Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E, for this reaction. Round your answer to 2 significant digits. kJ E x10 a mol IIarrow_forward
- Calculate the rate constant, k, for a reaction at 73.0 °C that has an activation energy of 77.2 kJ/mol and a frequency factor of 2.61 x 10¹¹ s-¹. k = X10 TOOLS $1arrow_forwardThe rate constant k for a certain reaction is measured at two different temperatures: temperature k 258.0 °C 3.9 x 1010 153.0 °C 1.5 × 1010 Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E, for this reaction. Round your answer to 2 significant digits. kJ E х10 a molarrow_forwardThe rate constant k for a certain reaction is measured at two different temperatures: temperature k 253.0 °C 7.6×10 ¹0 354.0 °C 5.8 × 10¹1 Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E for this reaction. a Round your answer to 2 significant digits. kJ E x10 mol X ? a II Śarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY