
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
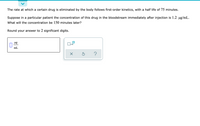
Transcribed Image Text:The rate at which a certain drug is eliminated by the body follows first-order kinetics, with a half life of 75 minutes.
Suppose in a particular patient the concentration of this drug in the bloodstream immediately after injection is 1.2 ug/mL.
What will the concentration be 150 minutes later?
Round your answer to 2 significant digits.
ug
x10
mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A decomposition reaction follows second order kinetics with a rate constant, k = 0.0137 M-1 min-1. If the initial concentration of the reactant is 0.866 M, what is the concentration after 5 minutes? Report your answer to the thousandths place and do not include units.arrow_forwardAt a certain temperature this reaction follows first-order kinetics with a rate constant of 0.0667 s¯¹: NH,OH (aq) → NH, (aq)+H,O (aq) Suppose a vessel contains NH4OH at a concentration of 0.360M. Calculate the concentration of NH4OH in the vessel 15.0 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. M x10 X ?arrow_forward5. You studied the chemical reaction, 2NO2(g) → 2NO(g) + O2(g), at 25°C by monitoring the concentration of NO2(g) as a function of time and constructed the following graph. y= 0.5408x + 125.0 R2=0.998 Time (s) What is the rate constant for this reaction at 25°C? Include the proper units. (W/T) [(3)ONI/Tarrow_forward
- Can someone please help with question 3arrow_forwardThe rate at which a certain drug is eliminated by the body follows first-order kinetics, with a half life of 69 minutes.Suppose in a particular patient the concentration of this drug in the bloodstream immediately after injection 1.9 is . What will the concentration be 207 minutes later? Round your answer to 2 significant digits.arrow_forwardA chemical engineer is studying the rate of this reaction. 2NH3 (g) → N₂ (g)+ 3H₂ (g) She fills a reaction vessel with NH3 and measures its concentration as the reaction proceeds. Here's a graph of her data: (W) [EHN] 0.20- 0.15- 0.10- 0.05- 0_ 20 60 t (s) 80 100arrow_forward
- 39. The decomposition of a certain pesticide was found to follow first-order kinetics. A student finds that after 3.0 months a sample of pesticide was 87.5% decomposed (only 12.5% of the pesticide remained). What is the half-life (112) for the decomposition reaction? a. 1.0 months b. 2.0 months 3.0 months d. The half-life can not be determined with the information provided.arrow_forwardQuestion 6 The rate at which a certain drug is eliminated by the body follows first-order kinetics, with a half life of 90 minutes. Suppose in a particular patient the concentration of this drug in the bloodstream immediately after injection is 0.19 μg/mL. What will the concentration be 180 minutes later? Round your answer to 2 significant digits.arrow_forwardIn the nuclear industry, workers use a rule of thumb that the radioactivity from any sample will be harmless after 10 half-lives. What fraction of a radioactive sample remains after this time? (Radioactive decay obeys first-order kinetics.)arrow_forward
- The rate at which a certain drug is eliminated by the body follows first-order kinetics, with a half life of 73 minutes. Suppose in a particular patient the concentration of this drug in the bloodstream immediately after injection is 1.1 ug/mL. What will the concentration be 365 minutes later? Round your answer to 2 significant digits. ug x10 mL ?arrow_forwardAt a certain temperature this reaction follows second-order kinetics with a rate constant of 64.6M¹s¹: NH,OH(aq) → NH, (aq)+H,O(aq) Suppose a vessel contains NH OH at a concentration of 0.170M. Calculate the concentration of NH OH in the vessel 0.920 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. 0 M x10 X S 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center 1 Accessibility Show Allarrow_forward60 QUESTION 13 The following graphs can be used for the questions that follow: 00 B0 00 Seris 1 02 time(min) D2 -D4 y = -0.0358x + 0.1058 Liner (Serive t) -18 time(min) The decomposition reaction of NO 2 is important in atmospheric chemistry. The reaction 2NO 2(g) → 2NO(g) + O 2(g) is second order with respect to NO 2. How can the rate constant be determined? A plot of 1/[NO2] versus time will be linear with a slope of -k. A plot of 1/[NO2] versus time will be linear with a slope of k. A plot of [NO2] versus time will be linear with a slope of -k. A plot of In[NO2] versus time will be linear with a slope of -k. Click Save and Submit to save and submit. Click Save All Answers to save all answers. MacBook Air DD F8 F3 F4 F5 F1 %23 24 2 4. 7.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY