
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
1. The radius of a rhodium atom is 134 pm. How many rhodium atoms would have to be laid side by side to span a distance of 1.70 mm?
2. The mass of a single uranium atom is 4.70×10-22 grams. How many uranium atoms would there be in 196 milligrams of uranium?
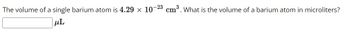
Transcribed Image Text:The volume of a single barium atom is 4.29 x 10-23 cm³. What is the volume of a barium atom in microliters?
μL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An atom has a diameter of 2.50 Å and the nucleus of that atom has a diameter of 6.00 x 10-5 Å. Determine the fraction of the volume of the atom that is taken up by the nucleus. Assume the atom and the nucleus are a sphere. fraction of atomic volume: Calculate the density of a proton, given that the mass of a proton is 1.0073 amu and the diameter of a proton is 1.69 × 10-¹5 density: m. g/cm³arrow_forward3) Atmospheric argon is a mixture of three stable isotopes 36Ar, 38Ar, and 40Ar. Use the information below to determine the atomic mass and natural abundance of 40Ar. The atomic weight of argon is 39.948 u. Argon-36 35.967545 u 0.337% Argon-38 37.96732 u 0.063% Argon-40 ? ?arrow_forwardThe planet Zoltan is located in a solar system in the Andromeda galaxy. On Zoltan, the standard unit for the amount of substance is the wog and the standard unit for mass is the wibble. The Zoltanians, like us, chose carbon-12 to define their standard unit for the amount of substance. By definition, one wog of C-12 atoms contains 2.50 x 1021 atoms. It has a mass of exactly 12 wibbles. What is the mass, in wibbles, of 1 wog of nitrogen atoms? What is the mass, in wibbles, of 5.00 x 10-1 wogs of O2? What is the mass, in grams of 1 wog of hydrogen atoms? Expert Solutionarrow_forward
- A fictitious element Z has an average atomic mass of 223.67 u.223.67 u. Element Z has two naturally occuring isotopes. The more abundant isotope has an exact mass of 224.29 u224.29 u and a relative abundance of 60.99%.60.99%. Calculate the exact mass of the second isotope.arrow_forwardThe radius of a tantalum atom is 142 pm. How many tantalum atoms would have to be laid side by side to span a distance of 1.87 mm? atomsarrow_forwardPlease do this chemistry workarrow_forward
- The radius of a strontium atom is 215 pm How many strontium atoms would have to be laid side by side to span a distance of 1.65 mm?arrow_forward9. Lithium hydroxide crystals are used in manned space vehicles to remove carbon dioxide gas from the air exhaled by the astronauts. The symbolic equation for this reaction is 2LIOH + CO₂ → Li₂CO3 + H₂O a. The formula and charge of a lithium ion is Lit Deduce the formula and charge of the carbonate ion. Explain your answer. b. A space vehicle carries a crew of 7 astronauts. Each astronaut exhales 18 moles of carbon dioxide every day. Calculate the total number of moles of carbon dioxide that the crew will exhale during a mission into space which lasts 10 days. Show your working. c. Calculate the mass of lithium hydroxide crystals which must be loaded on board the space vehicle to react with all the carbon dioxide exhaled during the mission. Show your working.arrow_forwardThe mass of a neutron is 1.6749×10−24 gg , and the mass of a proton is 1.6726×10−24 gg . What is the total mass of a neutron and a proton?arrow_forward
- 12.) Potassium (atomic mass= 39.10 amu) has three isotopes with masses 38.9367 amu, 39.9640amu, and 40. 9618 amu. The abundance of the last isotope is 6.73%. Estimate the abundances of the other two isotopes.arrow_forwardCalculate the total mass of the protons and electrons in fluorine-19. Use 1.007825 amu as the mass of hydrogen-1 (mass of a proton and an electron). Express your answer in atomic mass units to 6 significant figures.arrow_forwardPlease do this assignment based on on the image belowarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY