
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:9. Lithium hydroxide crystals are used in manned space vehicles to remove carbon dioxide gas from
the air exhaled by the astronauts.
The symbolic equation for this reaction is
2LIOH + CO₂ → Li₂CO3 + H₂O
a. The formula and charge of a lithium ion is Lit Deduce the formula and charge of the
carbonate ion.
Explain your answer.
b. A space vehicle carries a crew of 7 astronauts. Each astronaut exhales 18 moles of carbon
dioxide every day. Calculate the total number of moles of carbon dioxide that the crew
will exhale during a mission into space which lasts 10 days.
Show your working.
c. Calculate the mass of lithium hydroxide crystals which must be loaded on board the space
vehicle to react with all the carbon dioxide exhaled during the mission.
Show your working.
Expert Solution
arrow_forward
Step 1
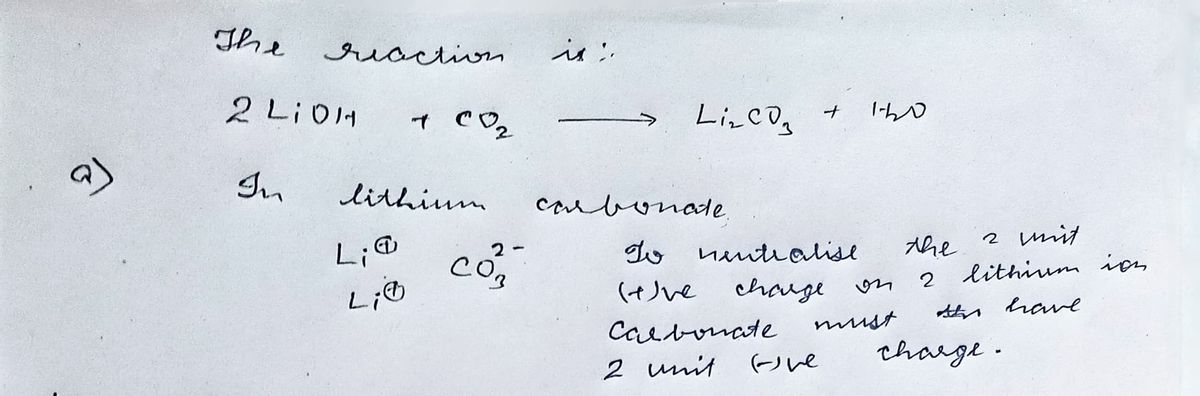
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4 The reaction between NH3 and O2 produces NO and H₂O. 4 NH3 (8) + 5 O₂ (g) 24 NO(g) + 6 H₂O (1) If a complete reaction of a mixture of NH3 and O₂ that had a total mass of 250 g produced 60 g of liquid water, what will be the most likely mass of all the gases remaining in the container after the reaction? Assume that reaction happens in a closed container. 190 g 60 g More information is needed to decide 310 garrow_forwardHow many moles of titanium are present in 124 g of titanium?arrow_forwardusing the equations provided, please help me figure out which of them goes to which problems which have also been providedarrow_forward
- Q7. What is the source of energy for stars and the sun. Explain the proton-proton fusion chain.arrow_forwardThe planet Zoltan is located in a solar system in the Andromeda galaxy. On Zoltan, the standard unit for the amount of substance is the wog and the standard unit for mass is the wibble. The Zoltanians, like us, chose carbon-12 to define their standard unit for the amount of substance. By definition, one wog of C-12 atoms contains 2.50 x 1021 atoms. It has a mass of exactly 12 wibbles. What is the mass, in wibbles, of 1 wog of nitrogen atoms? What is the mass, in wibbles, of 5.00 x 10-1 wogs of O2? What is the mass, in grams of 1 wog of hydrogen atoms? Expert Solutionarrow_forwardNa + 2 H2O à 2 NaOH + H2 If 50 g of sodium (Na) is placed in water, what mass of hydrogen gas (H2) is produced?arrow_forward
- Select the correct molecular mass of carbon monoxide (CO) by adding the atomic mass of carbon and the atomic mass of oxygen. A 22.4 L CO B 28.01 g CO U 55.85 g CO D 44.01 g COarrow_forwardC. The Born-Haber process is produces ammonia (NH3) and is responsible for the vast increase in agricultural production of the 20th century. Write and balance the equation for this process: diatomic nitrogen reacts with diatomic hydrogen to produce ammonia (NH3). 365 d. How many grams of diatomic nitrogen (N₂) are needed to produce 2.75 moles of ammonia?arrow_forwardRefer to the pictures:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY