
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
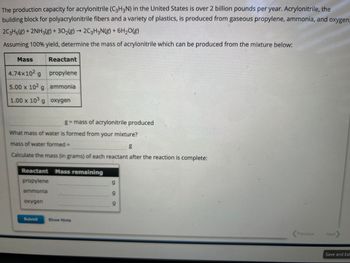
Transcribed Image Text:The production capacity for acrylonitrile (C3H3N) in the United States is over 2 billion pounds per year. Acrylonitrile, the
building block for polyacrylonitrile fibers and a variety of plastics, is produced from gaseous propylene, ammonia, and oxygen.
2C3H6(g) + 2NH3(g) + 302(g) → 2C3H3N(g) + 6H₂O(g)
Assuming 100% yield, determine the mass of acrylonitrile which can be produced from the mixture below:
Mass
4.74x102 g
5.00 x 102 g
1.00 x 103 g
Reactant
oxygen
propylene
ammonia
oxygen
What mass of water is formed from your mixture?
mass of water formed =
g
Calculate the mass (in grams) of each reactant after the reaction is complete:
Submit
g = mass of acrylonitrile produced
Reactant Mass remaining
propylene
ammonia
Show Hints
g
9
9
<Previous
Next
Save and Exi
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Cryolite (Na3AlF6) is used in the commercial production of aluminum from ore. Cryolite itself is produced by the following reaction: 6 NaOH + Al₂O3 + 12 HF → 2 Na3AlF6 + 9 H₂O A mixture containing 420.0 kg of NaOH, 228.4 kg of Al2O3, and 600.0 kg of HF is heated to 950 °C until it reacts to completion. What is the maximum mass of Na3AlF6 formed? kg Na3AlF6arrow_forwardThe human body burns glucose (C6H₁2O6) for energy according to this chemical reaction: C6H12O6 +60₂-6CO₂ + 6H₂O The products of the reaction are carbon dioxide (CO₂) and water (H₂O). Interestingly, all of the carbon dioxide and much of the water exits the body through the lungs: on every breath, the average person exhales 500. mL of air, which is typically enriched to 4% CO₂ and 5% water vapor by volume. In short, when a person loses weight by dieting, the weight that is lost actually departs his body as a gas, every time he exhales. Each kilogram of body fat lost requires exhaling about 2.9 kg of carbon dioxide. Calculate how many breaths it takes an average person to "exhale" 1.00 kg of fat. Round your answer to the nearest thousand. You'll need to know that the density of CO₂ is 2.0 kg/m³. 0 ☐ ☐x10 Xarrow_forwardChlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl(aq), as described by the chemical equation MnO2(s)+4HCl(aq)⟶MnCl2(aq)+2H2O(l)+Cl2(g) How much MnO2(s) should be added to excess HCl(aq) to obtain 285 mL Cl2(g) at 25 °C and 95 kPa?arrow_forward
- Consider the following reactions:CoO (s) + CO (g) D CO2 (g) + Co (s) Kc(1) = 490.2 CoO (s) + 2 H2 (g) D 2 Co (s) + 2 H2O (g) Kc(2) = 4.5 x 103a. Write the overall equation for the reaction of hydrogen gas and carbon dioxide gas to produce carbon monoxide gas and steam.arrow_forwardPotassium permanganate reacts with oxalic acid (H2C2O4) in aqueous sulfuric acid according to the following reaction: 2 KMnO4(aq) + 5 H2C2O4(aq) + 3 H2SO4(aq) 2 MnSO4·H2O(s) + K2SO4(aq) + 10 CO2(g) + 6 H2O(l) The reaction is run a second time and 8.74 g of MnSO4·H2O are obtained. What volume of carbon dioxide gas is collected at 1.01 bar and 23˚C? Answer in L with the correct number of significant figures; do not use scientific notation.arrow_forwardCryolite (Na3AlF6) is used in the commercial production of aluminum from ore. Cryolite itself is produced by the following reaction: 6 NaOH + Al₂O3 + 12 HF → 2 Na3AlF6 + 9 H₂O A mixture containing 430.0 kg of NaOH, 232.7 kg of Al2O3, and 600.0 kg of HF is heated to 950 °C until it reacts to completion. What is the maximum mass of Na3AlF6 formed? kg Na3AlF6arrow_forward
- Ammonia (NH3) is an important compountd is used in large amounts for the manufacture of nitrogenous fertilizers, nylon and many other important compounds. It is manufactured by the catalytic reaction between nitrogen between nitrogen and hydrogen, according to the following equation; N2(g) +H2(g) = NH;(g) When 1.20 mol nitrogen and 1.20 mol hydrogen are mixed together in a closed vessel, 30% of the nitrogen is converted into ammonia. a. Calculate the moles of nitrogen gas that will be present in the vessel? (only 3 decimal places) mol b. Calculate the moles of hydrogen gas that will be present in the vessel? (only 3 decimal places) mol c. Calculate the moles of ammonia gas that will be present in the vessel? (only 3 decimal places) Activate W mol d. What is the number of moles of nitrogen gas that has reacted? (only 3 decimal places) 10:09 Links 18°C 1O 23-Nov-21arrow_forwardChlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl(aq), as described by the chemical equation MnO2(s)+4HCl(aq)⟶MnCl2(aq)+2H2O(l)+Cl2(g) How much MnO2(s) should be added to excess HCl(aq) to obtain 115 mL Cl2(g) at 25 °C and 765 Torr? mass of MnO2: ______garrow_forwardCalcium hydride reacts with water to produce hydrogen gas according to the following reaction: CaH2(s) + 2 H2O(l) ----> 2H2(g) + Ca(OH)2(aq) This reaction is used to generate hydrogen gas to inflate air bags in cars and life rafts on boats and for similar uses where a simple compact means of hydrogen generation is necessary. Assuming complete reaction with water, how many grams of calcium hydride are required to fill a raft to a total pressure of 2.0 atm at 25oC if the volume of the raft is 1,000 L?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY