
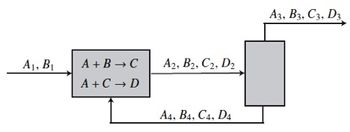
The process consists of two unit operations, a reactor and a separator. The process takes in some flow rates A1 and B1 of species A and B.
The reactor has a reaction that produces an intermediate product C that then needs to be converted to the desired product D by a second reaction.
A + B → C (Reaction 1)
A + C → D (Reaction 2)
The single-pass conversion of the reactor is 90% with a 30% selectivity for Reaction 2.
The products of the reaction in stream “2” are fed to a separator, which produces the product stream “3” and a recycle stream “4.”
The specifications of the separator are a recycle of 65% of D and 85% of C.
The flow B2 is evenly split between the product and recycle streams, while 10% of A2 is lost to the product stream.
Set up all relevant mass balances then develop the numerical methods required to study the output stream as a function of the inlet flow rates A1 and B1.
Briefly discuss your plan below.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

- REACTION ENGINEERING Consider a heterogeneous gas-phase catalytic reaction:AB(g)→A(g)+B(g) (Catalyst) The surface reaction mechanism follows three steps: AB(g) +S <----> AB*S (Adsorption)AB*S<----> B*S+A(g) (Surface reaction)B*S <---> B(g) + S (Desorption) (a) Derive the rate law assuming (1) surface reaction is rate limiting, and (2) desorption is rate-limiting. Express the overall reaction rate (- Tan) in terms of total number of sites on the surface, C. partial pressures of the gas species (ie., Pab Pa and Pb), rate constants of adsorption, the surface reaction, and desorption (ka,ks, and kd), and the equilibrium constants of adsorption, the surface reaction, and desorption (ie., Ka. Ks and Kd).arrow_forwardGiven the following reaction and using the data in the table, determine the order of the reaction:2NO2(g)→2NO(g)+O2(g) Time (sec) [NO2 ] ln [NO2 ] 1/[NO2 ] 0 0.0100 -4.605 100 100 0.00648 -5.039 154 200 0.00479 -5.341 209 300 0.00380 -5.573 263 400 0.00315 -5.760 317 500 0.00269 -5.918 372 600 0.00235 -6.057 426 a. none of these b. second c. zero d. third e. firstarrow_forwardH1arrow_forward
- s.) 7) The elementary reaction A+B → D is to be carried out to obtain a desired product (D). The rate constant for this reaction is k₁ = 150*exp(-5,000/T). Unfortunately, under conditions required for the reaction to occur, species B also undergoes a second-order decomposition to form an undesired product, B2U, with ry = k₂C, and k₂ = 300*exp(-2,000/T). 212 - a) What is the instantaneous selectivity for this system (SD/U)? Your answer must be simplified as much as possible. 4 Species: A, B, C, D, U YD = rD -VA ŶD= FD KD CA KU FAO-FA 4-02 b) Describe a reactor system (draw a schematic AND describe with words) and operating conditions you would use to maximize the selectivity to D.arrow_forwardProblem 3 Dibutyl phthalate (diester) is produced in a CSTR operated isothermally at 112°C. The rate of formation of the di-ester from phthalic anhydride and butanol being very fast, the controlling step, in the presence of sulphuric acid catalyst, is the conversion of mono-ester to the diester. The liquid phase reaction is: СЫН.(СООCН:)CООН + CН,ОН A C6H4(COOC4H6)2 + H2O D B The rate of the reaction is given by the expression -TA = kC; gmol/L.h where CA is the concentration of the mono-ester in gmol/L. The value of the apparent rate constant is given by: k = 0.7628 x 10ol1.965- L/gmol.h where T is the reaction temperature in K. The initial concentration of the mono-ester is 2.84 gmol/L and the density of the reaction mixture is 0.984 kg/L. (Molar mass of diester = 278) 1. In an existing plant consisting of a CSTR of 1.1 m diameter and 1.6 m height, the volumetric feed rate is 10 L/min. (a) Find the conversion achieved in an ideal CSTR. (b) If the conversion achieved in practice 68.5%…arrow_forwardwhen acetone is heated in the gas phase, decomposition into ketene and methane takes place according to: (CH3)2CO > CH2-CO + CH4 the reaction is irreversible and first order. The rate constant is 1.047 s-1 vid 725 C. Calculate the turnover of acetone obtained in an isothermal and isobaric tube reactor under the following operating conditions. The reactor is made up of 20 parallel connected tubes, each with a length of 10 meters and an inner diameter of 10 cm. The working pressure is 4 atm and the temperature is 725 C. The acetone supply amounts to 120 mol/s.arrow_forward
- Give two reasons (short statements) why the rate of a catalyzed reaction mightdecrease as the pressure of one of its products increases.arrow_forwardREACTION ENGINEERING Consider a heterogeneous gas-phase catalytic reaction:AB(g)→A(g)+B(g) (Catalyst) The surface reaction mechanism follows three steps: AB(g) +S <----> AB*S (Adsorption)AB*S<----> B*S+A(g) (Surface reaction)B*S <---> B(g) + S (Desorption) a) Derive the initial rates (AR) of the reaction, assuming there is no product (ie., A and B) in the feed (ie., PA Pao=0), assuming adsorption is the rate determining step. Use initial pressure of AB (ie., PARA), total number of sites on the surface (C), and the rate and equilibrium constants in the expressions.Hint: Based on the reaction mechanism, only AB and B are adsorbed on the surface, i.e., there is no evidence that A alone can be adsorbed on the surface.arrow_forwardQ2- The synthesis of ammonia proceeds according to the following reaction N₂ + 3 H₂ -----> 2 NH3 In a given plant, 4202 lb of nitrogen and 1046 lb of hydrogen are fed to the synthesis reactor per hour. Production of pure ammonia from this reactor is 3060 lb per hour. a. What is the limiting reactant. b. What is the percent excess reactant. c. What is the percent conversion obtained (based on the limiting reactant).arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





