
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Help me
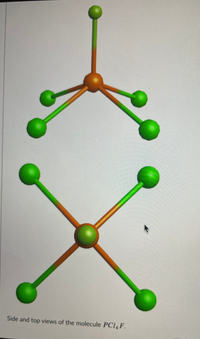
Transcribed Image Text:Side and top views of the molecule PCI4F.
![The Principal Axis is C, where n= [Select)
This molecule belongs to a "C" and not a "D" group because it lacks [Select ]
This molecule (Select]
>](https://content.bartleby.com/qna-images/question/67e70f86-06d8-4b86-a501-b94e483472a5/9a0406c3-fe0c-45b7-9d10-a120077710f7/b4cx61b_thumbnail.png)
Transcribed Image Text:The Principal Axis is C, where n= [Select)
This molecule belongs to a "C" and not a "D" group because it lacks [Select ]
This molecule (Select]
>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is An for the following equation in relating Ke toK 2 50 2 0 2g)2 50 30arrow_forwardHow can we increase the stability of droplet in technical DSMEarrow_forward15 16 17 18 19 20 21 22 23 24 25 26 27 An aqueous solution at 25 °C has a OH concentration of 1. x 10 "M. Calculate the H,O' concentration. Be sure your answer has 1 significant digits. -10 M dh Submit Assignment Continue MacBook Air F12 F9 F10 888 O00 FB 30 F7 F5 F6 esc F4 F2 F3 F1 & @ # $ %3D 4 5 7 8 9 2 3arrow_forward
- In each row check off the boxes that apply to the highlighted reactant. reaction + Ag+ (aq) + 2 NH3(aq) → Ag(NH3)2(aq) H₂(g) + Br₂(g) → 2 HBr(g) HNO₂(aq) + C₂H₂NH₂(aq) → NO₂(aq) + C₂H₂NH₂(aq) The highlighted reactant acts as a... (check all that apply) Brønsted-Lowry acid Brønsted-Lowry base Lewis acid Lewis base Brønsted-Lowry acid Brønsted-Lowry base Lewis acid Lewis base Brønsted-Lowry acid Brønsted-Lowry base Lewis acid Lewis basearrow_forwardcalculate the mass and volume (mL) of 5.0 mmol N,N-diethylethanolamine mw N,N-diethylethanolamine: 117.9g/mol density: 0.883 g/mLarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY