
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
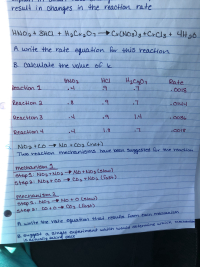
I need an explanation of #5

Expert Solution
arrow_forward
Step 1

Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A typical sports drink comes in a 20 fluid ounce bottle. 1 fluid ounce is 29.57 mL. Given your observed dye concentration, if you drank one bottle of sports drink per day for a year, how many grams of dye would you consume?arrow_forwardPlease answer question number 1 correctly with the correct answer. there is a picture of an example of a ''Dimensional analysis Format' below. please write out all work showing the math in dimensional analysis format. Question 3: Calculate the molar mass of each of the following ionic Compounds: A. KMnO4 B. Ca3(PO4)2arrow_forwardPart A Identify each of the labeled points (indicated with letters) or changes (indicated with two letters separated by an arrow) shown on the phase diagram. 1.00- Pressure (atm) 0.50- A 0.10- F -150 -75 75 150 Temperature ("C)arrow_forward
- A student dissolves 106 g of compound Q in one liter of hot water. All of compound Q dissolves. The student then cools the water to room temperature. At room temperature, 52 g of compound Q dissolves in one liter of water. How many grams of compound Q will recrystallize in the room temperature water? Include the unit in your answer.arrow_forwardArrange these substance according to how many drops we could add to a penny before the "bubble" popped, FEW DROPS (left-most) to MANY DROPS (right-most). The description labels on the images are small, so use the following legend to identify the materials: A: vegetable oil B: motor oil C: dish soap D: syrup E: honey A CITE EVIDENCE from your Learning Experience and provide REASONING for why you arranged these substances in this order.arrow_forwardCONCLUSIONS: In general, the boiling points of compounds increase down a group in the periodic table. The melting points and boiling points for the hydrogen compounds of group 6A elements are in the table below. Melting Point (°C) Boiling Point (°C) H₂O 0.0 100.0 H₂S -82.0 -60.0 H₂Se -65.7 -41.2 H₂Te -49.0 -2.2 Use your understanding of chemistry to propose an explanation for the anomaly in the trend for the hydrogen compounds of group 6A elements shown in the table.arrow_forward
- What is the following and what are the organic molecules? H H H C C Harrow_forwardhow to separate a solid mixture of lithium bromide and barium carbonatearrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY