
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
How do you solve question 1?
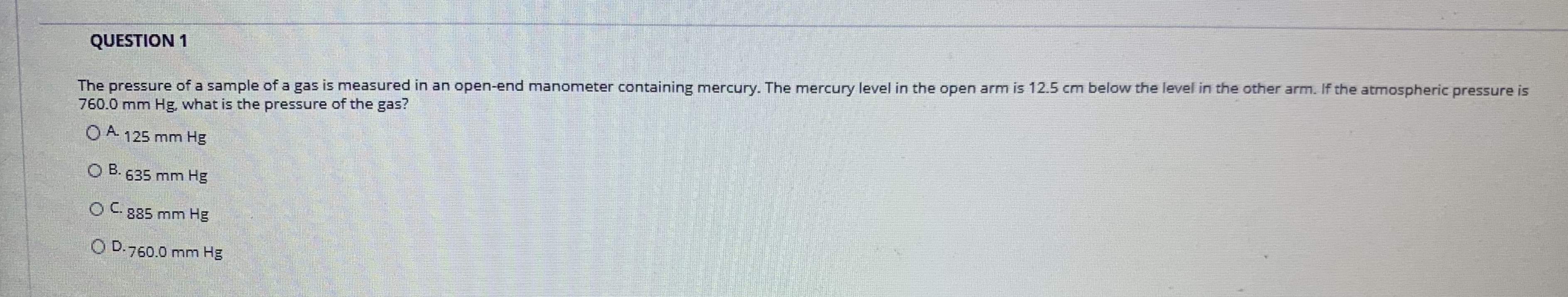
Transcribed Image Text:The pressure of a sample of a gas is measured in an open-end manometer containing mercury. The mercury level in the open arm is 12.5 cm below the level in the other arm. If the atmospheric pressure is
760.0 mm Hg, what is the pressure of the gas?
QUESTION 1
O A 125 mm Hg
B. 635 mm Hg
OC885 mm Hg
O D.760.0 mm Hg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you show us how to do number 8. ? Please see the attached pic.This is not a graded question as it is a practice question . I am 60 years old and helping my son prepare for the AP exam in a few months. We do questions at the back of the textbook by Zumdahl and Zumdahlarrow_forwardC-lab Answer the following questions. You may upload a handwritten file if you like, as long as it is legible. Part A: Figure numbers refer to figures in the lab procedures. Do the calculations in the units given. 1. Very briefly explain how a photogate works. 2. How far would you go if you traveled at a speed of 30 miles per hour for 3 hours? 3. What acceleration would be needed to travel the same distance in the same time as in question 2 when starting from rest? What would be your final speed? 4. Refer to Figure 3. Given a track length of 90 cm and a height difference of 18 cm, what is sin 0 5. Draw the force diagram for an object on an inclined plane (see Figure 2 in the lab manual). Attach the diagram in question 2 below. What would be the acceleration given the inclination from question 4 and g = 9.8 m/s? Part B: Attach the free body diagram from question 5 above. O 8:31 acer page up page dn & backspace delete 5 8 enterarrow_forwardWhat is green chemistry? Choose the BEST answer. O Chemistry that uses only recycled chemicals. O Chemistry that makes something green in color. O Chemistry that comes from plants or uses plant materials. O Chemistry that uses non-toxic, non-harmful chemicals.arrow_forward
- Every year Every second (1 year 365 days).arrow_forwardWhat is the difference between a direct and inverse relationship?arrow_forwardPlease answer question number 1 correctly with the correct answer. there is a picture of an example of a ''Dimensional analysis Format' below. please write out all work showing the math in dimensional analysis format. Question 2: What is the molar mass of each of the following compounds? A. Phosphorus Pentachloride (PCl5) B. Uranium Hexafluoride (UF6)arrow_forward
- 1 please assist with number one in the picture. I want to be we are correct. This is not a graded question as it is a practice question . I am 60 years old and helping my son prepare for the AP exam in a few months. We do questions at the back of the textbook by Zumdahl and Zumdahlarrow_forwardPlease answer both questions?arrow_forwardAnswer choices for blank 1(there is only one correct answer for each blank): A B C D E F G H J Answer choices for blank 2(there is only one correct answer for each blank): A B C D E F G H J Answer choices for blank 3(there is only one correct answer for each blank): A B C D E F G H J Answer choices for blank 4(there is only one correct answer for each blank): A B C D E F G H J Answer choices for blank 5(there is only one correct answer for each blank): A B C D E F G H Jarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY