
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
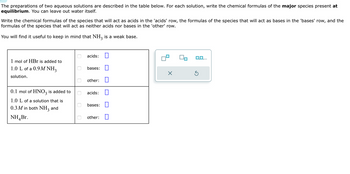
Transcribed Image Text:The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that NH3 is a weak base.
1 mol of HBr is added to
1.0 L of a 0.9M NH3
solution.
0.1 mol of HNO3 is added to
1.0 L of a solution that is
0.3 M in both NH3
and
NH Br.
0
0
0
0
0
acids: 0
bases:
other:
acids:
bases:
other:
X
S
Expert Solution
arrow_forward
Step 1: The main aim of the question
The objective of the question is to identify the species that will act as-
- Acids
- Bases
- Other
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 64 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCH₂CO₂ is a weak acid. 0.3 mol of NaOH is added to 1.0 L of a 0.3 M HCH₂ CO₂ solution. 0.13 mol of HCl is added to 1.0 L of a solution that is 1.3M in both HCH₂ CO₂ and KCH₂CO₂. acids: bases: other: acids: bases: other: × 0,0,... Śarrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH, is a weak base. acids: U D0.. 0.5 mol of HI is added to 1.0 L of a 0.5M NH3 bases: I solution. other: 0.07 mol of KOH is added to acids: 1.0 L of a solution that is 0.2M in both NH, and O. bases:U NH,Br. other: |arrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. 1 mol of HNO3 is added to 1.0 L of a 0.7M NH3 solution. 0.1 mol of KOH is added to 1.0 L of a solution that is 0.3M in both NH3 and NH₂ Br. acids: bases: other: acids: bases: other: 0 X 0,0,... Ś ? 09: allo 18 Ararrow_forward
- The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCN is a weak acid. acids: 0,0,.. 0.7 mol of KOH is added to 1.0 L of a 0.7M HCN bases: ? solution. other: U 0.1 mol of HNO, is added to acids: 1.0 L of a solution that is bases: 0.4M in both HCN and NaCN. other: Uarrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH, is a weak base. 0.7 mol of HNO, is O acids: O On added to 1.0 L of a bases: O 0.7 M NH, solution. ? O other: D 0.07 mol of HCl is added to 1.0 L of a solution that is 0.3 M in both NH, and acids: 0 bases: O O other: O NH,CI.arrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCH3CO₂ is a weak acid. 1 mol of NaOH is added to 1.0 L of a 0.6M HCH3 CO2 solution. 0.59 mol of HI is added to 1.0 L of a solution that is 1.2M in both HCH3CO₂ and KCH3CO2. acids: bases: other: 0 acids: bases: other: X 0,0,... Śarrow_forward
- The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCH3CO₂ is a weak acid. 0.4 mol of NaOH is added to 1.0 L of a 0.4MHCH, CO₂ solution. 0.1 mol of NaOH is added to 1.0 L of a solution that is 0.5M in both HCH, CO₂ and KCH, CO₂- □ acids: bases: other: acids: 0 bases: 17 other: X Śarrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. 1 mol of KOH is added to 1.0 L of a 0.6M HF solution. fn 0.3 mol of NaOH is added to 1.0 L of a solution that is 0.9M in both HF and KF. Explanation tab shift caps lock Check esc O acids: Obases: Oother: O 0 0 0 0 0 0 O acids: Obases: O Oother: O ! 1 A Q A Z 1 control option @ 2 A W S ►► # 3 X command E X D 00 $ 4 0.0.... 3 R F % 5 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility MacBook Pro 80 T 6 G Y B & 7 H U * 8 N J ( 9 4 7 K M ) O O A…arrow_forwardThe undissociated base (B) is present in the greatest concentration, with the products being present in relatively smaller amounts in this equilibrium mixture. How would this affect the magnitude of Kb? Do you expect it to be small or large? Which is the strongest base? Name of base Ammonia (NH3) Methylamine (CH3NH2) Ethylamine (C2H5NH2) Diethylamine (C2H5)2NH Pyridine (CSH5N) Kb value 1.76 x 10-5 Why? 4.4 x 10-4 5.6 x 10-4 Which is the weakest base? 1.3 x 10-3 1.7 x 10-9 Why? The larger the Kb the (stronger/weaker) the base. Since a weak base dissociates incompletely setting up an equilibrium, what method could you think of that you could use to determine the concentration of [OH"] ions given the initial concentration of the acid and its Kb?arrow_forward
- The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. 1 mol of KOH is added to 1.0 L of a 0.9M HF solution. 0.46 mol of KOH is added to 1.0 L of a solution that is 1.0M in both HF and NaF. U acids: bases: other: acids: bases: other: X 0,0,... Śarrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH, is a weak base. 0.26 mol of HCl is added to 1.0 L of a 1.2MNH₂ solution. 0.06 mol of HBr is added to 1.0 L of a solution that is 0.2M in both NH3 and NHẠC.. acids: bases: other: acids: bases: other: 1 09 X 8 0.0... Sarrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH, is a weak base. 1 mol of HNO3 is added to 1.0 L of a 0.6M NH3 solution.. 0.55 mol of NaOH is added to 1.0 L of a solution that is 1.5M in both NH3 and NH.CI. ☐ acids: ☐ ☐ bases: ☐ O other: O acids: ☐ bases: O other: 0° 0 0,0.... X 5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY