
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
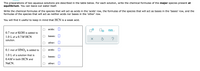
Transcribed Image Text:The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HCN is a weak acid.
acids:
0,0,..
0.7 mol of KOH is added to
1.0 L of a 0.7M HCN
bases:
?
solution.
other: U
0.1 mol of HNO, is added to
acids:
1.0 L of a solution that is
bases:
0.4M in both HCN and
NaCN.
other: U
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is a correct conjugate acid-base pair in the following reaction: NH3 + N₂H5+ NH4+ + N₂H4 Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a b с d NH3, N₂H4 + NH3, NH4* NH4+, N₂H5+ NH4+, N₂H4 X Your answerarrow_forwardA ph of 5 is how many times more acidic tham a ph of 8?arrow_forwardAssign each species on the left to a category on the right. Clear All (C,Hg),NH strong acid Ba(OH)2 weak acid CH3COOH strong base НСООН weak base Previousarrow_forward
- L. For the following chemical reaction, identify each reactant as an acid or base. Also, identify each product as a conjugate acid or base. NH3 + HCI ->>> NH4+ CIarrow_forwardIn a triprotic acid, which Ka has the highest value? Select one: O a. Ka1 O b. Ka2 О с. Каз O d. Kb1 e. Кр2arrow_forwardDraw the most likely conjugate base resulting from this acid-base reaction. Include all lone pairs. Ignore inorganic byproducts. :O: :0: NaOEt Drawin Qarrow_forward
- For the system below, if the position of the equilibrium lies to the LEFT. Which is the strongest acid in the system? CH3NH2 + NH3OH+ ⇌ NH2OH + CH3NH3+ Question options: CH3NH3+ CH3NH2 CH3NH2 and NH3OH+ are equally strong acids NH3OH+ NH2OHarrow_forwardUsing the Brønsted theory, classify the compounds as either an acid or a base. C,H,OH CH,CH,O Acid CH,CH,NH Answer Bank Basearrow_forwardIdentify the weaker acid in each of the following groups. I: HIO3, HIO2 Il: H20, H2S II: HBRO, HCIO HIO3 H2S, HCIO HIO2 H2S, HBRO HIO2 H20, HBro HIO3 H20, HBroarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY