
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
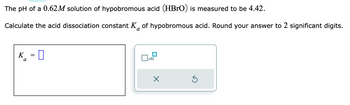
Transcribed Image Text:The pH of a 0.62M solution of hypobromous acid (HBrO) is measured to be 4.42.
Calculate the acid dissociation constant K of hypobromous acid. Round your answer to 2 significant digits.
K =
a
||
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
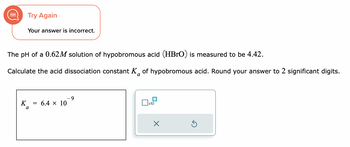
Transcribed Image Text:Try Again
Your answer is incorrect.
The pH of a 0.62M solution of hypobromous acid (HBrO) is measured to be 4.42.
Calculate the acid dissociation constant K of hypobromous acid. Round your answer to 2 significant digits.
a
K =
a
6.4 × 10
9
x10
X
S
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question

Transcribed Image Text:Try Again
Your answer is incorrect.
The pH of a 0.62M solution of hypobromous acid (HBrO) is measured to be 4.42.
Calculate the acid dissociation constant K of hypobromous acid. Round your answer to 2 significant digits.
a
K =
a
6.4 × 10
9
x10
X
S
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- G The pH of a 0.18M solution of hypobromous acid (HBrO) is measured to be 4.69. Calculate the acid dissociation constant K of hypobromous acid. Round your answer to 2 significant digits. K₁ = ⇓) x10arrow_forwardCalculate the pH and percent ionization of a 0.940 M HNO₂ solution. Note: Reference the K of acids at 25 °C table for additional information. a Part 1 of 2 Be sure your answer has the correct number of significant digits. pH= 1.72 Part 2 of 2 2.04 % x10 Be sure your answer has the correct number of significant digits. x10 × ×arrow_forwardThe pH of a 0.28 M solution of hypobromous acid (HBrO) is measured to be 4.60. Calculate the acid dissociation constant K of hypobromous acid. Round your answer to 2 significant digits. K.-0 X Sarrow_forward
- A chemist dissolves 163. mg of pure nitric acid in enough water to make up 360. mL of solution. Calculate the pH of the solution. Round your answer to 3 significant decimal places. 0 x10 X 5arrow_forwardThe pH of a 0.49M solution of acrylic acid (HC₂H₂CO₂) is measured to be 2.28. Calculate the acid dissociation constant K of acrylic acid. Round your answer to 2 significant digits. a K₁ = 0 x10 X Śarrow_forwardComplete the table below, Be sure each of your answer entries has the correct number of significant digits. You may assume the temperature is 25 °C. formula H.CO, HBrO conjugate acid CHINHY K₂ 45 × 10 ** 23 x 10 23 x 10 formula HCO conjugate base K Bro CHÍNH, A 2.2 × 10 43 × 10 44 × 10 % 0° 0.9 Xarrow_forward
- Calculate the pH of a 0.060 M C₂H5N (pyridine) solution. The K, for pyridine is 1.7 x 10. Be sure your answer has the correct number of significant digits. pH = 0 x10 Xarrow_forwardThe pH of a 1.2M solution of hexanoic acid (HC H,,0,) is measured to be 2.39. Calculate the acid dissociation constant K, of hexanoic acid. Be sure your answer has the correct number of significant digits. K a x10arrow_forwardO ACIDS AND BASES Calculating the pH of a weak base solution 0/3 The base protonation constant ₂ of trimethylamine ((CH³)²N) ¡S 6.31×10¯³. Calculate the pH of a 0.27 M solution of trimethylamine at 25 °C. Round your answer to 1 decimal place. pH = 0 Xarrow_forward
- Fill in the missing chemical formulae in the tables below. acid conjugate base base conjugate acid H₂O H₂O HCl H₂O + ☐ ☐ So 2- 4 ப OH ☐arrow_forwardThe chemical formulae of some acids are listed in the first column of the table below, and in the second column it says whether each acid is strong or weak. Complete the table. List the chemical formula of each species present at concentrations greater than about 10-6 mol/L when about a tenth of a mole of the acid is dissolved in a liter of water. strong or weak? species present at 106 mol/L or greater when dissolved in water acid HBr strong HC10, weak HBro, strong HF weak The chemIcarTomuae or sor Complete the table. List the e is dissolved in a liter of waterarrow_forwardComplete the table below. Round each of your entries to 2 significant digits. You may assume the temperature is 25 °C. conjugate acid conjugate base x10 formula K, formula K, CH,NH, 4 4.4 x 10 HCH,CO, - 5 1.8 x 10 H BrO -9 2.3 x 10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY