
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
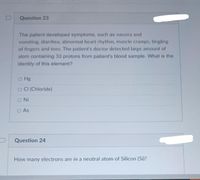
Transcribed Image Text:Question 23
The patient developed symptoms, such as nausea and
vomiting, diarrhea, abnormal heart rhythm, muscle cramps, tingling
of fingers and toes. The patient's doctor detected large amount of
atom containing 33 protons from patient's blood sample. What is the
identity of this element?
o Hg
O CI (Chloride)
ONi
O As
Question 24
How many electrons are in a neutral atom of Silicon (Si)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pls help ASAP on all PLS I REQUESTarrow_forwardComplete the table below, using the diagram of an atom shown at right. name symbol 0 0 proton Continue e 0 Properties of subatomic particles approximate mass (amu) (choose one) charge (in multiples of e) 0 0 7 (choose one) (choose one) O location on diagram (choose one) O (choose one) O (choose one) X 0 S W Hill LLC All Rights Reserved. Terms of Use Earrow_forwardPlease don't provide handwritten solution ....arrow_forward
- Ular) react together to produce new The first step in writing balanced chemical reactions is identifying the substances in the reaction and labeling them as elements, molecular compounds or ionic compounds. Based on the type of substance it is, you then write the correct formula from the given name. Element: a single type of atom or a diatomic element. Recognized in the description as a single element name by itself. Elements are always neutral (they have no charge). Check if a diatomic element. Molecular compound: made of two nonmetal atoms. Greek prefixes in the name indicate the number of each type of atom in the formula. lonic compound: made of a cation and anion. Recognized because they start with a metal or ammonium. Need the charges of the ions to determine the formula of the neutral compound. For each chemical name below label it as an element (E), molecular (M), or ionic (I) and then write the correct formula for the substance on the line next to it. 1. Aluminum reacts with…arrow_forwardDon't provide handwriting solutionsarrow_forwardOn earth, tin has 10 different stable isotopes. The heaviest, 124 Sn makes up 5.80% of naturally occurring tin atoms. a) How many atoms of 124 Sn are present in 42.7 g of a natural sample of tin? 124 Sn: 1.491e24 atoms Scientific Notation: 1.491 x 1024 Helpful tip: To enter a number in scientific notation, enter 0E5 or 1.0e5, for 1.0 x 105 where the E# (or e# ) denotes 10# b) Considering 124 Sn has an isotopic mass of 123.905 amu, calculate the mass of this isotope in the sample. Mass: 8.0 garrow_forward
- Symbol Abundance in Nature Protons: Periodic Table Neutrons: Electrons: H. Не Li Be C Ne My Isotope Symbol Carbon-13 Abundance in Nature +1 Stable O Mass Number 13 Neutrons Atomic Mass (amu)arrow_forwardPlease try to solve the questions 3 and 4 on the picturearrow_forwardif a gold atom has a radius of 145 pm and you could string gold atoms like beads on a thread, how many atoms would you need to have a necklace 36 cm longarrow_forward
- Hi! I am on the study section that my teacher has provided to help us prepare for our test tomorrow if we choose to use it! I am having a hard time with this question and would like to get a better understanding before the test tomorrow.arrow_forwardQuestion 37 of 39 Drop Calculated charge (el) 6.44 x 10-14 el 2.30 x 10-14 el C 3.22 x 10-14 el 1.84 x 10-14 el Given the results of the scientist's experiment, determine the charge on an electron in electrinos. electron charge: el Given that the charge on an electron is 1.60 x 10-19 C, determine the conversion factor between electrinos and coulombs. 1C = el 15 A tv P warrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY