
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
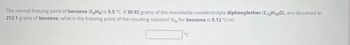
Transcribed Image Text:The normal freezing point of benzene (C6H6) is 5.5 °C. If 30.92 grams of the nonvolatile nonelectrolyte diphenylether (C12H100), are dissolved in
212.1 grams of benzene, what is the freezing point of the resulting solution? Kfp for benzene is 5.12 °C/m.
°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Shown below is the titration curve for phosphoric acid. At what pH is the solution entirely in the H3PO4 form? 0-0.5 14 12.5 O2.1 NICHarrow_forwardA solution with a density of 0.876 g>mL contains 5.0 g of toluene 1C7H82 and 225 g of benzene. Calculate the molarity of the solution.arrow_forwardHow many moles of KBr are there in 89.5 mL of 0.150 M KBr?arrow_forward
- Calculate the molarity (M) of a 65% (w/v) copper sulfate solution (m.w. = 160.0 g/mol).arrow_forwardHow many grams of sucrose (molecular weight = 342 g mole−1) would you dissolve in water to make a 0.22 M sucrose solution with 1 L final volume?arrow_forwardThe half-life of mercury-197 is 64.1 hours. If a patient undergoing a kidney scan is given 5.0 ng of mercury-197, how much will remain after 7 days? After 30 days?arrow_forward
- The following data give the constant dissociation, Ka (for acetic acid at various temperatures): T(°C) 0 Ка 1.657 x 10-5 10 1.729 x 10-5 15 1.745 x 10-5 25 1.753 × 10-5 kJ ΔΗ° = 1.53 mol Submit Previous Answers Part B Correct Plot Inka vs. 1/T and fit a line to the points. The slope will correspond to —▲H° / R. 1/T InKa 3.66 x 10-3-11.01 3.53 x 10-3-10.97 3.47 x 10-3-10.96 3.36 × 10-3-10.95 InKa = (−184 K) (±) — 10.3 = slope -184 K = AH° R AH° -184 K = 0.008314 kJ/(mol·K) AH 1.53 kJ/mol = Use these data, and the constant dissociation at 25 °C, to calculate AS° for acetic acid ionization. (InK Express your answer with the appropriate units. μÅ AS° = Value Units ? -AH° + ᎡᎢ. AS° Rarrow_forwardWhat is the pH of a buffer solution that is 0.20 M proprionic acid (HC3H4O2) and 0.1 M sodium proprionate (NaC3H4O2)? The KA of proprionic acid is 1.3 x 10-5arrow_forwardIn order to make a solution of the concentration 0.364 M, what mass of NaCl should bedissolved in 152 mL of water?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON