
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
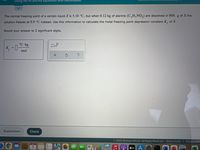
Transcribed Image Text:Using the Kt and Kb equations with electrolytes
The normal freezing point of a certain liquid X is 5.10 °C, but when 0.12 kg of alanine (C,H,NO,) are dissolved in 800. g of X the
solution freezes at 0.9 °C instead. Use this information to calculate the molal freezing point depression constant K, of X.
Round your answer to 2 significant digits.
°C-kg
K, =
mol
Explanation
Check
O 2022 McGraw Hill LLC. All Rights Reserved.
Terms of Use
Privacy Cent
FEB
3.
étv A
Expert Solution
arrow_forward
Step 1
given, the mass of C3H7NO2 = 0.12kg = 0.12 g
mass of solvent X = 800. g = 0.800 kg
molar mass of C3H7NO2 = 89 g mol-1
freezing point of liquid X = 5.10 oC
freezing point of solution = 0.9 oC
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The freezing point of water is 0.00°C at 1 atmosphere. How many grams of manganese(II) sulfate (151.0 g/mol), must be dissolved in 273.0 grams of water to reduce the freezing point by 0.350°C ? Refer to the table for the necessary boiling or freezing point constant. Solvent Formula Kp (°C/m) Kf(°C/m) Water H20 0.512 1.86 Ethanol CH3CH2OH 1.22 1.99 Chloroform CHC13 3.67 Benzene C6H6 2.53 5.12 Diethyl ether CH3CH2OCH,CH3 2.02 g manganese(II) sulfate.arrow_forwardWhat are the boiling point and freezing point of a 1.47 m solution of naphthalene in benzene? Note: Reference the Molal freezing point depression and boiling point elevation constants table for additional information. Part 1 of 2 Round your answer to 4 significant digits. Boiling point = Part 2 of 2 °C X Round your answer to 3 significant digits. Freezing point = X 3arrow_forwardA certain liquid X has a normal freezing point of -5.00 °C and a freezing point depression constant K, = 2.11 °C kg-mol ¹. A solution is prepared by dissolving some zinc chloride (ZnCl₂) in 800. g of X. This solution freezes at -6.7 °C. Calculate the mass of ZnCl, that was dissolved. Round your answer to 2 significant digits. x10 Sarrow_forward
- Determine the freezing point of a solution that contains 78.8 g of naphthalene (molar mass 128.16) dissolved in 722 mL of benzene (d= .877 g/mL). Pure benzene has a melting point of 5.50 degrees Celsius and a freezing point depression constant of 4.90 degrees Celsius/ marrow_forward- 1 The molal boiling point elevation constant K,=0.88 °C·kg•mol (NH.),CO) (NH, CÓ) are dissolved in 650. g of X, the for a certain substance X. When 67. g of urea solution boils at 115.2 °C. Calculate the boiling point of pure X. Round your answer to 4 significant digits. x10arrow_forwardThe normal boiling point of a certain liquid X is 110.00 °C, but when 106. g of alanine (C3H,NO₂) are dissolved in 900. g of X the solution boils at 111.9 °C instead. Use this information to calculate the molal boiling point elevation constant K, of X. Round your answer to 2 significant digits. K₁=0 °C.kg mol X Sarrow_forward
- What observable characteristics are common in the solutions? What substance does this characteristic offer to the solution?arrow_forwardAt a certain temperature the vapor pressure of pure benzene (C6H6) is measured to be 0.68 atm. Suppose a solution is prepared by mixing 88.0 g of benzene and 64.1 g of thiophene (C4H4S). Calculate the partial pressure of benzene vapor above this solution. Round your answer to 2 significant digits. Note for advanced students: you may assume the solution is ideal. 0 atm 0x10 Xarrow_forwardA certain liquid X has a normal freezing point of 4.10 °C and a freezing point depression constant K,=2.77 °C-kg-mol¯¹. Calculate the freezing point of a solution made of 40.g of sodium chloride (NaCl) dissolved in 800. g of X. Round your answer to 1 significant digit. [°C x10 X Śarrow_forward
- please help with the following questiongarrow_forwardA certain liquid X has a normal freezing point of -9.00 °C and a freezing point depression constant K, = 7.04 °C-kg mol . A solution is prepared by dissolving some potassium bromide (KBr) in 400. g of X. This solution freezes at -13.6 °C. Calculate the mass of KBr that was dissolved. Round your answer to 2 significant digits.arrow_forwardThe normal boiling point of a certain liquid X is 91.9 °C, but when 86.4 g of urea ((NH₂), CO) are dissolved in 850. g of X, it is found that the solution boils at 94.0 °C instead. Use this information to calculate the molal boiling point elevation constant K, of X. Round your answer to 2 significant digits. C-mol¹-kg 0.2 Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY