
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
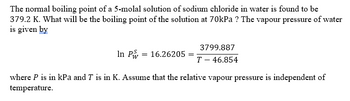
Transcribed Image Text:The normal boiling point of a 5-molal solution of sodium chloride in water is found to be
379.2 K. What will be the boiling point of the solution at 70kPa ? The vapour pressure of water
is given by
In P
=
16.26205
3799.887
T - 46.854
where P is in kPa and T is in K. Assume that the relative vapour pressure is independent of
temperature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Choose from the choices and show complete solution with clear explanation please, thank you.arrow_forwardFor substance A, it is known that the vapor pressure at 300K is 100kPa. It is also known that the boiling point temperature is 340K at 150 kPa. Other given information about substance A: CpL= 200 J/mol.K and Cpv = 180 J/mol.K a) Develop a vapor pressure correlation for substance A of the form In(P) = B – (A/T). b) For the correlation developed in part a) to be dimensionally consistent, state the units of the constants A and Barrow_forward3. Carbon dioxide gas of upstream conditions T, = 456.3 K, P = 59.04 bar is throttled to a downstream pressure of 1.0 bar. Use the Lee/Kesler generalized method to estimate the downstream temperature to a first approximation. (R = 8.314 J/mol-K). %3D %3D %3D For carbon dioxide: T = 394.2 K; Pc = 73.8 bar; w = 0.224 %3D %3D 2 = A + BT + DT-2 R A = 5.457 103B = 1.045 %3D %3D 10-5D = -1.157 %3Darrow_forward
- i need some help wuth this question.please help.arrow_forward“A liquid is heated at constant pressure in a smooth container to a temperature above its boiling point, at which it becomes superheated and metastable. Boiling chips are added and the liquid transforms irreversibly into a vapor phase. Why does the relationship Δs = Δh/T not describe the change in entropy for this particular transition?”arrow_forwardProblem 1.2: The equation below gives the boiling temperature of isopropanol as a function of pressure: B A - log10 P where T is in kelvin, P is in bar, and the parameters A, B, and Care A = 4.57795, B = 1221.423, C=-87.474. 1.6 Problems T = C₁ Obtain an equation that gives the boiling temperature in °F, as a function of In P, with P in psi. Hint: The equation is of the form B' A' - In P but the constants A', B', and C' have different values from those given above. T= 27arrow_forward
- 2. At a temperature of 400°C, the specific enthalpy of a water sample is 3100 KJ/kg. What is the phase of the water? a. Solid b. Solid-liquid mixture c. Subcooled liquid d. Saturated liquid e. Saturated mixture f. Saturated vapor g. Superheated vapor For the water in problem 2, determine the following: a. If saturated mixture calculate the quality, x = b. Determine the pressurearrow_forwardThis table displays the vapor pressure of nitrogen at several different temperatures. Use the data to determine the heat of vaporization and normal boiling point of nitrogen.Temperature (K) Pressure (torr)65 130.570 289.575 570.880 102885 1718arrow_forwardThe standard enthalpy of combustion of solid phenol, C6H5OH, is 3054 kJ mol-1 at 298 K and its standard molar entropy is 144.0 J K-1 mol-1 . Use these values, together to calculate the standard Gibbs energy of formation of phenol at 298 K.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The