
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
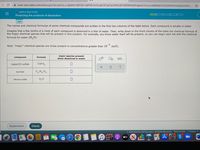
Transcribed Image Text:www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLqkt1FLlq7wcPWKzBYGFE9IMFJEfhQpHb7nCnyvQOBWafV32ZDMTAHmarGpUzh4V..
O SIMPLE REACTIONS
Predicting the products of dissolution
The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water.
Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of
the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical
formula for water (H,O).
Note: "major" chemical species are those present in concentrations greater than 10
9-
mol/L.
major species present
when dissolved in water
compound
formula
copper(II) sulfate
Cuso,
C12 H2, O11
sucrose
nitrous oxide
N,0
Explanation
Check
© 2021 McGraw-Hill Education. All Riahts Reserved. Terms of Use | Privacy
d山T國
tv
占の
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H2O).arrow_forwardA solution is made by dissolving 29.3 g of aluminum chloride, AlCl3, in enough water to make exactly 100 mL of solution. Calculate the concentration (molarity) of AlCl3 in mol/L (M).arrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). -6 Note: "major" chemical species are those present in concentrations greater than 10 mol/L. compound isopropanol fructose zinc iodide formula C₂H₂O C6H₁2O6 12 ZnI, major species present when dissolved in water 0 × 0,0,... Śarrow_forward
- Matti is given two bottles of copper(II) sulfate solutions in her senior high chemistry lab. She is told that one bottle contains a saturated solution and the other one contains an unsaturated solution. The solutions are the exact same color and have no particles in the bottles. Describe what Matti can do to distinguish between the two solutions.arrow_forwardExpand the units of molarity for this solution: 0.035 M CaCl2 When the units are expanded, enter the terms that appear in the numerator and the denominator, in the proper order. Do not leave any blank empty. Enter the chemical formula like we have previously. Use the following abbreviations as needed: moles = mol, liters = L, solution = soln Numerator: Denominator:arrow_forwardYou dissolve 6.64 mol of NaCI into a total volume of 6.91 L. What is the molarity of NaCI in the resulting solution. (Give your answer in units of M, with two places after the decimal point.)arrow_forward
- Calculate the Normality of Hcl of a solution that contains 45 g in 325 ml of water, knowing that the Molecular Mass of the Acid is 26.4 g / mol. Density is 1.19 g / ml and its purity is 27%.arrow_forwardWhat are the similarities and differences between the everyday and technical meanings and uses of the terms insoluble and solubility ?arrow_forwardSugar and table salt (sodium chloride) are both soluble in water. Sugar is a molecular compound and table salt is an ionic compound. Select the correct statements below. An aqueous solution of sugar can conduct electricity Pure water can conduct electricity An aqueous solution of table salt cannot conduct electricity An aqueous solution of table salt can conduct electricityarrow_forward
- The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). Note: "major" chemical species are those present in concentrations greater than 10 -9- mol/L. dlo major species present when dissolved in water compound formula glycerol C,H,O, isopropanol C,H,0 nickel(II) chloride NiCl,arrow_forwardExpand the units of molarity for this solution: 0.58 M AGNO3 When the units are expanded, enter the terms that appear in the numerator and the denominator, in the proper order. Do not leave any blank empty. Enter the chemical formula like we have previously. Use the following abbreviations as needed: moles = mol, liters = L, solution = soln Numerator: Denominator:arrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). Note: "major" chemical species are those present in concentrations greater than 10 mol/L. -6 compound iron(II) sulfate iron(II) iodide ammonium iodide formula FeSO4 FeI₂ NH₂I major species present when dissolved in water Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY